granisetron-hydrochloride
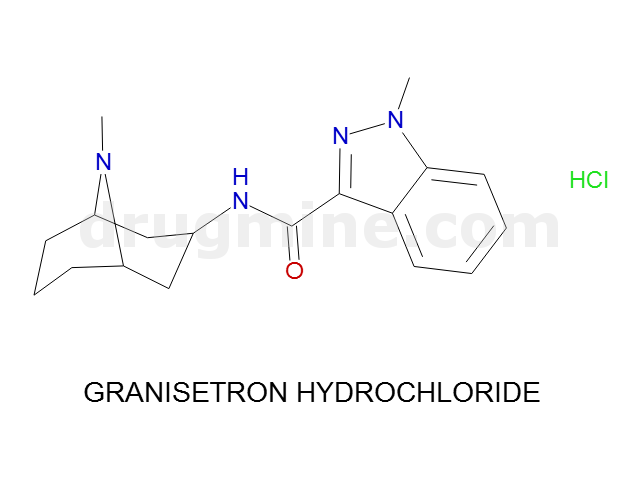

Name: GRANISETRON HYDROCHLORIDE
ID :
MW: 312
Number of atoms: 23
Molecular_Formula: C18H24N4O
Alogp: 2.401
Indication class : Anti-Emetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
granisetron hydrochloride containing products summary
There are in total 57 different products containing the active ingredient granisetron hydrochloride. From the 57 drug products, 14 have been discontinued.Product id = 8670
Application Number = 20239
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3MG BASE/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8671
Application Number = 20239
Date of Application = 11,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8668
Application Number = 20239
Date of Application = 17,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 8669
Application Number = 20239
Date of Application = 11,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROCHE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 23191
Application Number = 20305
Date of Application = 16,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 23192
Application Number = 20305
Date of Application = 15,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 13863
Application Number = 21238
Date of Application = 27,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = KYTRIL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/10ML
----
Product id = 7996
Application Number = 77165
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7977
Application Number = 77177
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7970
Application Number = 77186
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7971
Application Number = 77187
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7990
Application Number = 77297
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21999
Application Number = 77842
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7969
Application Number = 77913
Date of Application = 26,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7989
Application Number = 77963
Date of Application = 3,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 21993
Application Number = 78037
Date of Application = 27,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 22001
Application Number = 78080
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 1MG BASE
----
Product id = 7976
Application Number = 78090
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7995
Application Number = 78096
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7964
Application Number = 78197
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7965
Application Number = 78198
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7966
Application Number = 78198
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21992
Application Number = 78221
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7973
Application Number = 78258
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7974
Application Number = 78258
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21994
Application Number = 78260
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7972
Application Number = 78262
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 13826
Application Number = 78334
Date of Application = 28,, Feb, 2008
RX/OTC/DISCN = DISCN
Tradename = GRANISOL
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PEDIATRX
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/10ML
----
Product id = 7988
Application Number = 78392
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7975
Application Number = 78522
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7984
Application Number = 78531
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7985
Application Number = 78531
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7982
Application Number = 78534
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7992
Application Number = 78564
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7993
Application Number = 78565
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7991
Application Number = 78566
Date of Application = 29,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7997
Application Number = 78629
Date of Application = 23,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = GRANISTERON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7998
Application Number = 78629
Date of Application = 23,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = GRANISTERON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21998
Application Number = 78678
Date of Application = 13,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 21996
Application Number = 78725
Date of Application = 30,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7983
Application Number = 78808
Date of Application = 29,, Apr, 2008
RX/OTC/DISCN = DISCN
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7986
Application Number = 78835
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7987
Application Number = 78835
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21991
Application Number = 78843
Date of Application = 27,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 21995
Application Number = 78846
Date of Application = 27,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7967
Application Number = 78863
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7994
Application Number = 78863
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7968
Application Number = 78880
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 21997
Application Number = 78969
Date of Application = 22,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7962
Application Number = 79078
Date of Application = 14,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7963
Application Number = 79078
Date of Application = 14,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7961
Application Number = 79119
Date of Application = 10,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 22000
Application Number = 90817
Date of Application = 28,, May, 2010
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 7980
Application Number = 91136
Date of Application = 9,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.1MG BASE/ML (EQ 0.1MG BASE/ML)
----
Product id = 7981
Application Number = 91136
Date of Application = 9,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 7979
Application Number = 91137
Date of Application = 9,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT AGILA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 7978
Application Number = 91274
Date of Application = 22,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = GRANISETRON HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----