flutamide
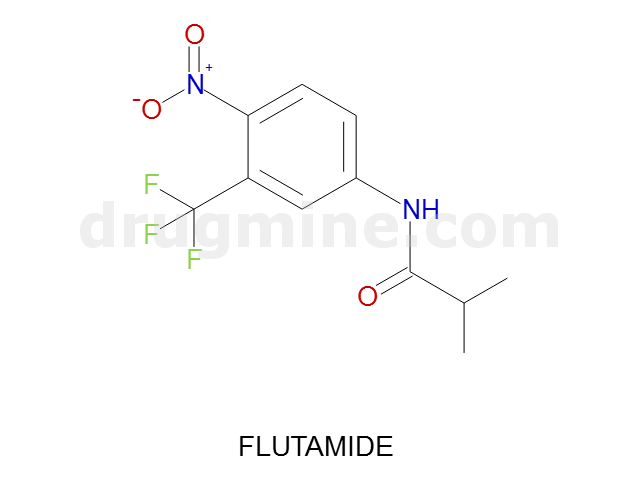
Name: FLUTAMIDE
ID :
MW: 276
Number of atoms: 19
Molecular_Formula: C11H11F3N2O3
Alogp: 2.916
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
flutamide containing products summary
There are in total 6 different products containing the active ingredient flutamide. From the 6 drug products, 2 have been discontinued.Product id = 1893
Application Number = 18554
Date of Application = 27,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = EULEXIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 2042
Application Number = 75298
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = FLUTAMIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG
----
Product id = 2040
Application Number = 75780
Date of Application = 19,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = FLUTAMIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 125MG
----
Product id = 2043
Application Number = 75818
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = FLUTAMIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 2039
Application Number = 75820
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = FLUTAMIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG
----
Product id = 2041
Application Number = 76224
Date of Application = 9,, May, 2003
RX/OTC/DISCN = RX
Tradename = FLUTAMIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG
----