flurbiprofen
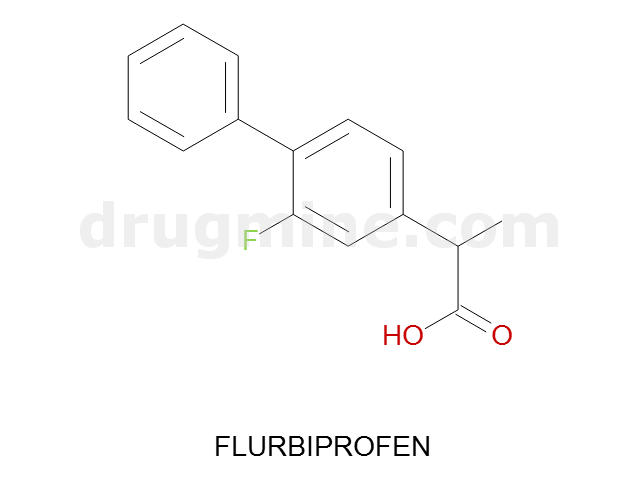
Name: FLURBIPROFEN
ID :
MW: 244
Number of atoms: 18
Molecular_Formula: C15H13FO2
Alogp: 3.68
Indication class : Anti-Inflammatory; Analgesic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
flurbiprofen containing products summary
There are in total 16 different products containing the active ingredient flurbiprofen. From the 16 drug products, 9 have been discontinued.Product id = 18032
Application Number = 18766
Date of Application = 31,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = ANSAID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18033
Application Number = 18766
Date of Application = 31,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = ANSAID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 21461
Application Number = 74358
Date of Application = 20,, Jun, 1994
RX/OTC/DISCN = RX
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21462
Application Number = 74358
Date of Application = 20,, Jun, 1994
RX/OTC/DISCN = RX
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21470
Application Number = 74405
Date of Application = 24,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21469
Application Number = 74405
Date of Application = 24,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21459
Application Number = 74411
Date of Application = 31,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21460
Application Number = 74411
Date of Application = 31,, May, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21471
Application Number = 74431
Date of Application = 31,, May, 1995
RX/OTC/DISCN = RX
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 21465
Application Number = 74448
Date of Application = 28,, Jul, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21466
Application Number = 74448
Date of Application = 28,, Jul, 1995
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21472
Application Number = 74560
Date of Application = 16,, May, 1997
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THERAGEN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21463
Application Number = 74647
Date of Application = 1,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 21464
Application Number = 74647
Date of Application = 1,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 21467
Application Number = 75058
Date of Application = 27,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 21468
Application Number = 75058
Date of Application = 27,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = FLURBIPROFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----