flumazenil
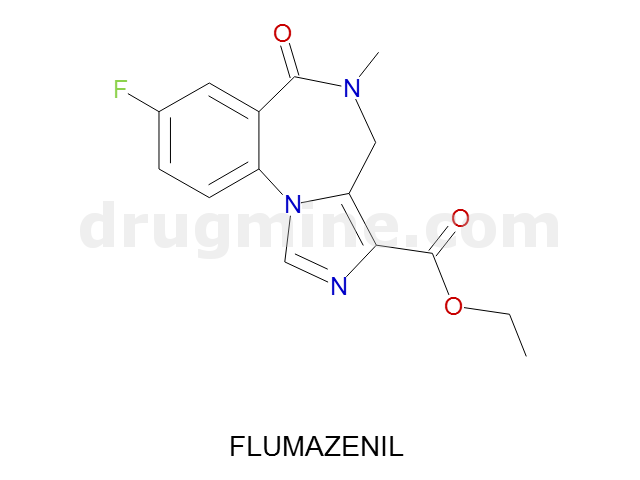


Name: FLUMAZENIL
ID :
MW: 303
Number of atoms: 22
Molecular_Formula: C15H14FN3O3
Alogp: 1.583
Indication class : Antagonist (to benzodiazepine)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
flumazenil containing products summary
There are in total 20 different products containing the active ingredient flumazenil. From the 20 drug products, 6 have been discontinued.Product id = 10699
Application Number = 20073
Date of Application = 20,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = ROMAZICON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/10ML (0.1MG/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 10700
Application Number = 20073
Date of Application = 20,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = ROMAZICON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML) **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 7685
Application Number = 76256
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7684
Application Number = 76256
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7697
Application Number = 76589
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7696
Application Number = 76589
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7683
Application Number = 76755
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7682
Application Number = 76755
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7691
Application Number = 76787
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7690
Application Number = 76787
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7687
Application Number = 76955
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7686
Application Number = 76955
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7694
Application Number = 77071
Date of Application = 3,, May, 2005
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7695
Application Number = 77071
Date of Application = 3,, May, 2005
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7688
Application Number = 78527
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7689
Application Number = 78527
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7680
Application Number = 78595
Date of Application = 13,, May, 2008
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7681
Application Number = 78595
Date of Application = 13,, May, 2008
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 1MG/10ML (0.1MG/ML)
----
Product id = 7692
Application Number = 90584
Date of Application = 28,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.5MG/5ML (0.1MG/ML)
----
Product id = 7693
Application Number = 90584
Date of Application = 28,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = FLUMAZENIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 002
Tecode = AP
Rld = No
Strength = 1MG/10ML (0.1MG/ML)
----