fenofibrate
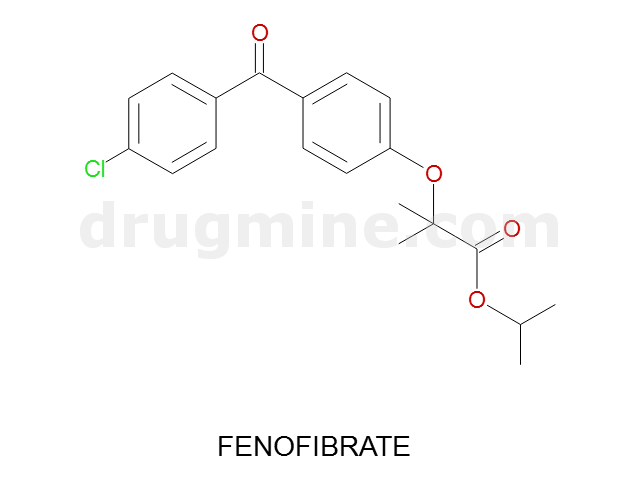


Name: FENOFIBRATE
ID :
MW: 361
Number of atoms: 25
Molecular_Formula: C20H21ClO4
Alogp: 5.111
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fenofibrate containing products summary
There are in total 53 different products containing the active ingredient fenofibrate. From the 53 drug products, 10 have been discontinued.Product id = 2360
Application Number = 19304
Date of Application = 31,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = LIPIDIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 3652
Application Number = 19304
Date of Application = 9,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = TRICOR (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = No
Strength = 67MG
----
Product id = 3653
Application Number = 19304
Date of Application = 30,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = TRICOR (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = No
Strength = 134MG
----
Product id = 3654
Application Number = 19304
Date of Application = 30,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = TRICOR (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 29166
Application Number = 21203
Date of Application = 4,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = TRICOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 54MG
----
Product id = 29167
Application Number = 21203
Date of Application = 4,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = TRICOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBOTT
ProductNo = 003
Tecode =
Rld = No
Strength = 160MG
----
Product id = 29195
Application Number = 21350
Date of Application = 7,, May, 2005
RX/OTC/DISCN = DISCN
Tradename = TRIGLIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SKYEPHARMA AG
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29196
Application Number = 21350
Date of Application = 7,, May, 2005
RX/OTC/DISCN = RX
Tradename = TRIGLIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SKYEPHARMA AG
ProductNo = 002
Tecode = BX
Rld = Yes
Strength = 160MG
----
Product id = 2361
Application Number = 21612
Date of Application = 11,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = LIPOFEN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 2362
Application Number = 21612
Date of Application = 11,, Jan, 2006
RX/OTC/DISCN = DISCN
Tradename = LIPOFEN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 2363
Application Number = 21612
Date of Application = 11,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = LIPOFEN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = CIPHER PHARMS INC
ProductNo = 003
Tecode =
Rld = Yes
Strength = 150MG
----
Product id = 29168
Application Number = 21656
Date of Application = 5,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = TRICOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 48MG
----
Product id = 29169
Application Number = 21656
Date of Application = 5,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = TRICOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 145MG
----
Product id = 1088
Application Number = 21695
Date of Application = 30,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = ANTARA (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 43MG
----
Product id = 1089
Application Number = 21695
Date of Application = 30,, Nov, 2004
RX/OTC/DISCN = DISCN
Tradename = ANTARA (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 87MG
----
Product id = 1091
Application Number = 21695
Date of Application = 30,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = ANTARA (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 130MG
----
Product id = 1087
Application Number = 21695
Date of Application = 18,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ANTARA (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 004
Tecode =
Rld = No
Strength = 30MG
----
Product id = 1090
Application Number = 21695
Date of Application = 18,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ANTARA (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 90MG
----
Product id = 21254
Application Number = 22118
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FENOGLIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21255
Application Number = 22118
Date of Application = 10,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = FENOGLIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 120MG
----
Product id = 1930
Application Number = 75753
Date of Application = 3,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 67MG
----
Product id = 1931
Application Number = 75753
Date of Application = 9,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 134MG
----
Product id = 1932
Application Number = 75753
Date of Application = 9,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 1922
Application Number = 75868
Date of Application = 27,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 67MG
----
Product id = 1923
Application Number = 75868
Date of Application = 27,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 134MG
----
Product id = 1924
Application Number = 75868
Date of Application = 27,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21250
Application Number = 76433
Date of Application = 13,, May, 2005
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 54MG
----
Product id = 21251
Application Number = 76433
Date of Application = 13,, May, 2005
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 160MG
----
Product id = 21238
Application Number = 76509
Date of Application = 26,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 54MG
----
Product id = 21239
Application Number = 76509
Date of Application = 26,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 21242
Application Number = 76520
Date of Application = 25,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 54MG
----
Product id = 21243
Application Number = 76520
Date of Application = 29,, Dec, 2005
RX/OTC/DISCN = DISCN
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 107MG
----
Product id = 21244
Application Number = 76520
Date of Application = 25,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 21247
Application Number = 76635
Date of Application = 31,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 54MG
----
Product id = 21248
Application Number = 76635
Date of Application = 31,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode =
Rld = No
Strength = 107MG
----
Product id = 21249
Application Number = 76635
Date of Application = 31,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 160MG
----
Product id = 21252
Application Number = 90715
Date of Application = 5,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VALEANT INTL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 48MG
----
Product id = 21253
Application Number = 90715
Date of Application = 5,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VALEANT INTL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 145MG
----
Product id = 21240
Application Number = 90856
Date of Application = 23,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 48MG
----
Product id = 21241
Application Number = 90856
Date of Application = 23,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 145MG
----
Product id = 1920
Application Number = 90859
Date of Application = 1,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS SA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 43MG
----
Product id = 1921
Application Number = 90859
Date of Application = 1,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS SA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 130MG
----
Product id = 1916
Application Number = 201748
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 43MG
----
Product id = 1917
Application Number = 201748
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 130MG
----
Product id = 1918
Application Number = 202252
Date of Application = 26,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 43MG
----
Product id = 1919
Application Number = 202252
Date of Application = 26,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 130MG
----
Product id = 1925
Application Number = 202579
Date of Application = 10,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 43MG
----
Product id = 1927
Application Number = 202579
Date of Application = 10,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 130MG
----
Product id = 1926
Application Number = 202676
Date of Application = 23,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 67MG
----
Product id = 1928
Application Number = 202676
Date of Application = 23,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 134MG
----
Product id = 1929
Application Number = 202676
Date of Application = 23,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE (MICRONIZED)
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 21245
Application Number = 202856
Date of Application = 7,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 48MG
----
Product id = 21246
Application Number = 202856
Date of Application = 7,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = FENOFIBRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 145MG
----