etoposide
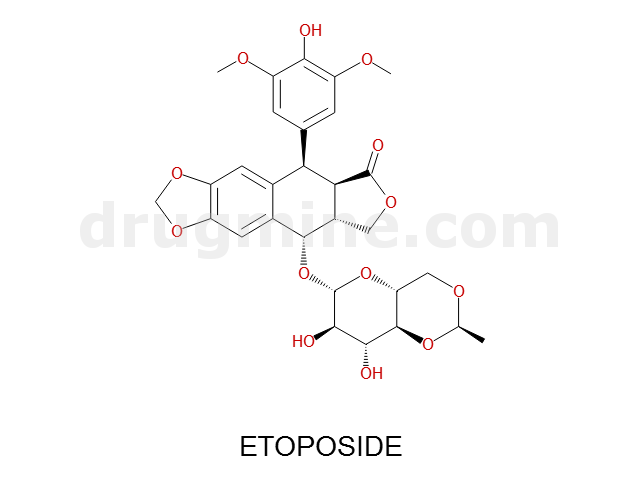

Name: ETOPOSIDE
ID :
MW: 589
Number of atoms: 42
Molecular_Formula: C29H32O13
Alogp: 0.935
Indication class : Antineoplastic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
etoposide containing products summary
There are in total 17 different products containing the active ingredient etoposide. From the 17 drug products, 12 have been discontinued.Product id = 11354
Application Number = 18768
Date of Application = 10,, Nov, 1983
RX/OTC/DISCN = DISCN
Tradename = VEPESID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = CORDEN PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 3713
Application Number = 19557
Date of Application = 30,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = VEPESID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 3714
Application Number = 19557
Date of Application = 30,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = VEPESID
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 11124
Application Number = 74166
Date of Application = 27,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = TOPOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7565
Application Number = 74227
Date of Application = 22,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMACHEMIE BV
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7570
Application Number = 74228
Date of Application = 15,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7568
Application Number = 74284
Date of Application = 10,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7561
Application Number = 74290
Date of Application = 17,, Jul, 1995
RX/OTC/DISCN = RX
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 7563
Application Number = 74320
Date of Application = 30,, Aug, 1995
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7564
Application Number = 74351
Date of Application = 30,, Aug, 1995
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7567
Application Number = 74510
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7560
Application Number = 74513
Date of Application = 14,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 7569
Application Number = 74529
Date of Application = 24,, Jul, 1996
RX/OTC/DISCN = RX
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 7566
Application Number = 74813
Date of Application = 9,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PIERRE FABRE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7571
Application Number = 74968
Date of Application = 9,, Jan, 1998
RX/OTC/DISCN = DISCN
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/ML
----
Product id = 7562
Application Number = 74983
Date of Application = 30,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ETOPOSIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 1892
Application Number = 75635
Date of Application = 19,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ETOPOSIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG
----