efavirenz
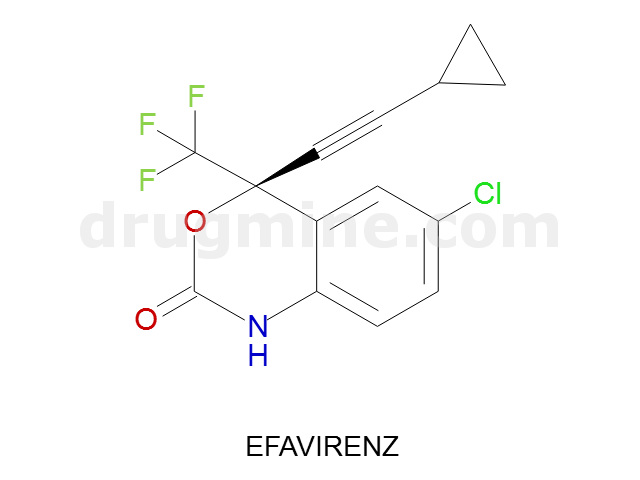
Name: EFAVIRENZ
ID :
MW: 316
Number of atoms: 21
Molecular_Formula: C14H9ClF3NO2
Alogp: 4.381
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
efavirenz containing products summary
There are in total 6 different products containing the active ingredient efavirenz. From the 6 drug products, 2 have been discontinued.Product id = 3355
Application Number = 20972
Date of Application = 17,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = SUSTIVA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 3356
Application Number = 20972
Date of Application = 17,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = SUSTIVA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 3357
Application Number = 20972
Date of Application = 17,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = SUSTIVA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 28419
Application Number = 21360
Date of Application = 1,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = SUSTIVA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 28420
Application Number = 21360
Date of Application = 1,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = SUSTIVA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = Yes
Strength = 600MG
----
Product id = 18211
Application Number = 21937
Date of Application = 12,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = ATRIPLA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GILEAD
ProductNo = 001
Tecode =
Rld = Yes
Strength = 600MG;200MG;300MG
----