dipyridamole
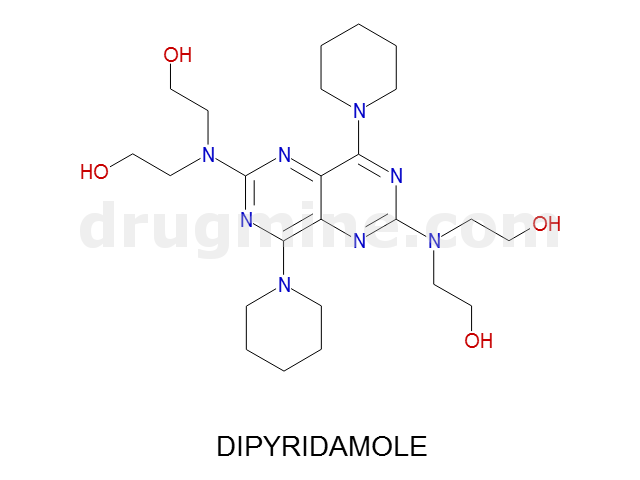
Name: DIPYRIDAMOLE
ID :
MW: 505
Number of atoms: 36
Molecular_Formula: C24H40N8O4
Alogp: 2.483
Indication class : Vasodilator (coronary)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
dipyridamole containing products summary
There are in total 40 different products containing the active ingredient dipyridamole. From the 40 drug products, 16 have been discontinued.Product id = 26127
Application Number = 12836
Date of Application = 22,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = PERSANTINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 26128
Application Number = 12836
Date of Application = 6,, Feb, 1987
RX/OTC/DISCN = RX
Tradename = PERSANTINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 004
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 26129
Application Number = 12836
Date of Application = 6,, Feb, 1987
RX/OTC/DISCN = RX
Tradename = PERSANTINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 75MG
----
Product id = 8552
Application Number = 19817
Date of Application = 13,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = IV PERSANTINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 310
Application Number = 20884
Date of Application = 22,, Nov, 1999
RX/OTC/DISCN = RX
Tradename = AGGRENOX
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;200MG
----
Product id = 20537
Application Number = 40542
Date of Application = 21,, Apr, 2006
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20538
Application Number = 40542
Date of Application = 21,, Apr, 2006
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20539
Application Number = 40542
Date of Application = 21,, Apr, 2006
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 20534
Application Number = 40733
Date of Application = 13,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MURTY PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20535
Application Number = 40733
Date of Application = 13,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MURTY PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20536
Application Number = 40733
Date of Application = 13,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MURTY PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 20528
Application Number = 40782
Date of Application = 18,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20529
Application Number = 40782
Date of Application = 18,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20530
Application Number = 40782
Date of Application = 18,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 20547
Application Number = 40874
Date of Application = 28,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20548
Application Number = 40874
Date of Application = 28,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20549
Application Number = 40874
Date of Application = 28,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 20531
Application Number = 40898
Date of Application = 23,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20532
Application Number = 40898
Date of Application = 23,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20533
Application Number = 40898
Date of Application = 23,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 7280
Application Number = 74521
Date of Application = 18,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5MG/ML
----
Product id = 7281
Application Number = 74601
Date of Application = 19,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 7278
Application Number = 74939
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 5MG/ML
----
Product id = 7282
Application Number = 74952
Date of Application = 26,, Nov, 1997
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 7279
Application Number = 74956
Date of Application = 30,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5MG/ML
----
Product id = 7277
Application Number = 75769
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 322
Application Number = 78804
Date of Application = 14,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = ASPIRIN AND DIPYRIDAMOLE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;200MG
----
Product id = 20543
Application Number = 86944
Date of Application = 16,, Apr, 1991
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20546
Application Number = 87160
Date of Application = 7,, Jun, 1996
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20522
Application Number = 87184
Date of Application = 3,, Oct, 1990
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20545
Application Number = 87561
Date of Application = 25,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 20544
Application Number = 87562
Date of Application = 25,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20523
Application Number = 87716
Date of Application = 3,, Oct, 1990
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 20524
Application Number = 87717
Date of Application = 3,, Oct, 1990
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 20525
Application Number = 88999
Date of Application = 5,, Feb, 1991
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20526
Application Number = 89000
Date of Application = 5,, Feb, 1991
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20527
Application Number = 89001
Date of Application = 5,, Feb, 1991
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 20540
Application Number = 89425
Date of Application = 12,, Jul, 1990
RX/OTC/DISCN = RX
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 20541
Application Number = 89426
Date of Application = 12,, Jul, 1990
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20542
Application Number = 89427
Date of Application = 12,, Jul, 1990
RX/OTC/DISCN = DISCN
Tradename = DIPYRIDAMOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----