diclofenac-sodium
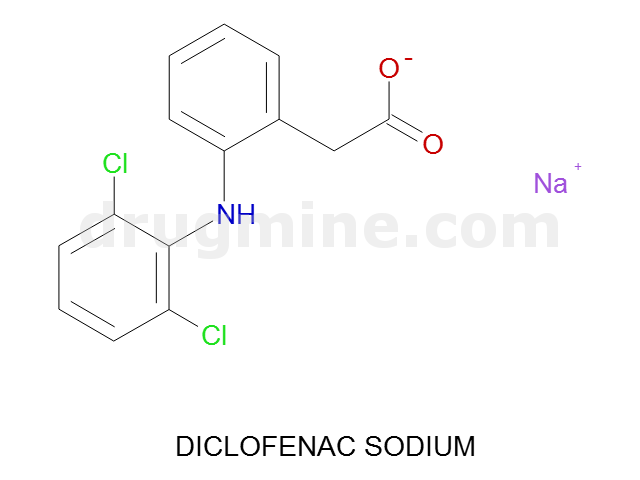


Name: DICLOFENAC SODIUM
ID :
MW: 295
Number of atoms: 19
Molecular_Formula: C14H10Cl2NO2
Alogp: 2.899
Indication class : Anti-Inflammatory
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
diclofenac sodium containing products summary
There are in total 58 different products containing the active ingredient diclofenac sodium. From the 58 drug products, 17 have been discontinued.Product id = 15611
Application Number = 19201
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = VOLTAREN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 15612
Application Number = 19201
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = VOLTAREN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15613
Application Number = 19201
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = VOLTAREN
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 13197
Application Number = 20037
Date of Application = 28,, Mar, 1991
RX/OTC/DISCN = RX
Tradename = VOLTAREN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.1%
----
Product id = 16775
Application Number = 20254
Date of Application = 8,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = VOLTAREN-XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 15395
Application Number = 20607
Date of Application = 24,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = ARTHROTEC
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;0.2MG
----
Product id = 15396
Application Number = 20607
Date of Application = 24,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = ARTHROTEC
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 75MG;0.2MG
----
Product id = 12935
Application Number = 20809
Date of Application = 4,, May, 1998
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = FALCON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 14265
Application Number = 20947
Date of Application = 4,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PENNSAID
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = NUVO RES INC
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 1.5%
----
Product id = 5433
Application Number = 21005
Date of Application = 16,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = SOLARAZE
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 3%
----
Product id = 5447
Application Number = 22122
Date of Application = 17,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = VOLTAREN
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1%
----
Product id = 13553
Application Number = 22396
Date of Application = 23,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = DYLOJECT
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = JAVELIN PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 37.5MG/ML (37.5MG/ML)
----
Product id = 15425
Application Number = 74376
Date of Application = 28,, Sep, 1995
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG
----
Product id = 15426
Application Number = 74376
Date of Application = 28,, Sep, 1995
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 50MG
----
Product id = 15429
Application Number = 74390
Date of Application = 15,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15422
Application Number = 74391
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 15423
Application Number = 74391
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15424
Application Number = 74391
Date of Application = 29,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15427
Application Number = 74394
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 75MG
----
Product id = 15420
Application Number = 74432
Date of Application = 29,, Jul, 1999
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15421
Application Number = 74432
Date of Application = 29,, Jul, 1999
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15430
Application Number = 74459
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 15431
Application Number = 74459
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15432
Application Number = 74459
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15411
Application Number = 74514
Date of Application = 26,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15412
Application Number = 74514
Date of Application = 26,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 15428
Application Number = 74723
Date of Application = 30,, Mar, 1999
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15418
Application Number = 74986
Date of Application = 26,, Feb, 1999
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 15419
Application Number = 74986
Date of Application = 26,, Feb, 1999
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 75MG
----
Product id = 15415
Application Number = 75185
Date of Application = 13,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 15413
Application Number = 75185
Date of Application = 13,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 15414
Application Number = 75185
Date of Application = 13,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15416
Application Number = 75281
Date of Application = 12,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15417
Application Number = 75281
Date of Application = 12,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 15865
Application Number = 75492
Date of Application = 11,, Feb, 2000
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT INTL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 15862
Application Number = 75910
Date of Application = 7,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 15864
Application Number = 76152
Date of Application = 13,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 15863
Application Number = 76201
Date of Application = 6,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DEXCEL LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 12933
Application Number = 77600
Date of Application = 13,, Nov, 2008
RX/OTC/DISCN = DISCN
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12931
Application Number = 77845
Date of Application = 17,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 15435
Application Number = 77863
Date of Application = 8,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 75MG
----
Product id = 12932
Application Number = 78031
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12936
Application Number = 78553
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = NEXUS PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12934
Application Number = 78792
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 15433
Application Number = 90066
Date of Application = 1,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 15434
Application Number = 90066
Date of Application = 1,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15440
Application Number = 200158
Date of Application = 9,, May, 2013
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;0.2MG
----
Product id = 15441
Application Number = 200158
Date of Application = 9,, May, 2013
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 75MG;0.2MG
----
Product id = 15438
Application Number = 200540
Date of Application = 14,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = EAGLE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;0.2MG
----
Product id = 15439
Application Number = 200540
Date of Application = 14,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = EAGLE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 75MG;0.2MG
----
Product id = 5390
Application Number = 200936
Date of Application = 28,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 3%
----
Product id = 15436
Application Number = 201089
Date of Application = 9,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;0.2MG
----
Product id = 15437
Application Number = 201089
Date of Application = 9,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM AND MISOPROSTOL
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 75MG;0.2MG
----
Product id = 14160
Application Number = 202027
Date of Application = 27,, May, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1.5%
----
Product id = 14161
Application Number = 202393
Date of Application = 24,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1.5%
----
Product id = 14163
Application Number = 202852
Date of Application = 24,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1.5%
----
Product id = 14162
Application Number = 203818
Date of Application = 26,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = DICLOFENAC SODIUM
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1.5%
----
Product id = 14264
Application Number = 204623
Date of Application = 16,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = PENNSAID
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = HORIZON PHARMA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2%
----