desonide
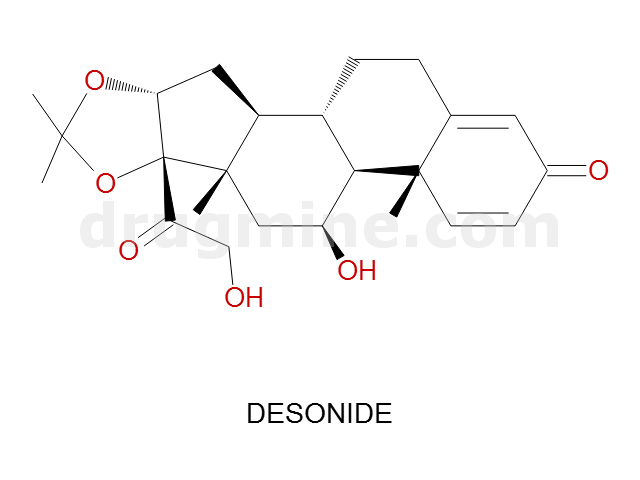
Name: DESONIDE
ID :
MW: 417
Number of atoms: 30
Molecular_Formula: C24H32O6
Alogp: 1.028
Indication class : Anti-Inflammatory
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
desonide containing products summary
There are in total 14 different products containing the active ingredient desonide. From the 14 drug products, 2 have been discontinued.Product id = 4114
Application Number = 17010
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 12442
Application Number = 17426
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 13241
Application Number = 17914
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIDESILON
Route/format = OTIC / SOLUTION/DROPS
Application Type = N
Applicant Name = BAYER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2%;0.05%
----
Product id = 4117
Application Number = 19048
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = DESOWEN
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 5387
Application Number = 21844
Date of Application = 20,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = DESONATE
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.05%
----
Product id = 17
Application Number = 21978
Date of Application = 19,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = VERDESO
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = N
Applicant Name = AQUA PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.05%
----
Product id = 12444
Application Number = 71425
Date of Application = 15,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = DESOWEN
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12189
Application Number = 72354
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = RX
Tradename = DESOWEN
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 4115
Application Number = 73548
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 4116
Application Number = 74027
Date of Application = 28,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = DESONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 12443
Application Number = 74254
Date of Application = 3,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12441
Application Number = 75751
Date of Application = 12,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12187
Application Number = 75860
Date of Application = 19,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12188
Application Number = 202161
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = DESONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----