desogestrel
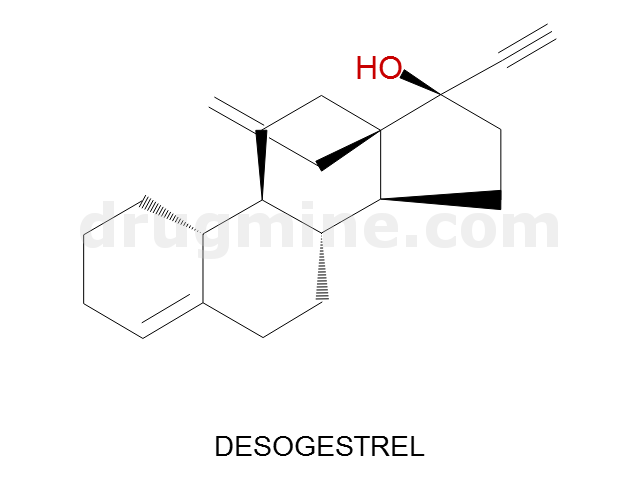
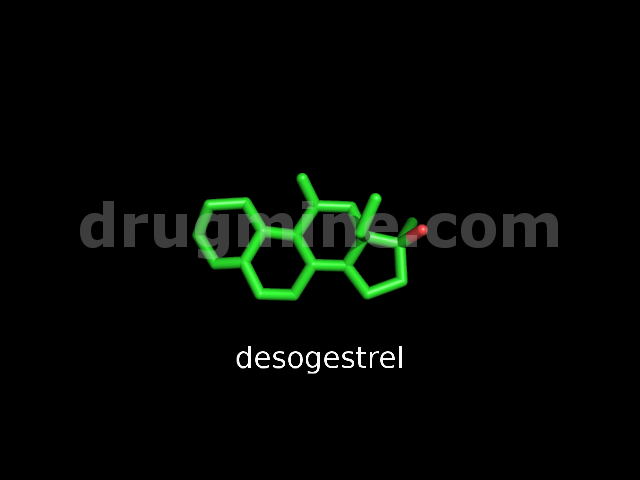
Name: DESOGESTREL
ID :
MW: 310
Number of atoms: 23
Molecular_Formula: C22H30O
Alogp: 5.828
Indication class : Progestin
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
desogestrel containing products summary
There are in total 19 different products containing the active ingredient desogestrel. From the 19 drug products, 4 have been discontinued.Product id = 29907
Application Number = 20071
Date of Application = 10,, Dec, 1992
RX/OTC/DISCN = DISCN
Tradename = DESOGEN
Route/format = ORAL-21 / TABLET
Application Type = N
Applicant Name = ORGANON USA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29992
Application Number = 20071
Date of Application = 10,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DESOGEN
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = ORGANON USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29954
Application Number = 20301
Date of Application = 14,, Dec, 1992
RX/OTC/DISCN = DISCN
Tradename = ORTHO-CEPT
Route/format = ORAL-21 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 30092
Application Number = 20301
Date of Application = 14,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = ORTHO-CEPT
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 0.15MG;0.03MG
----
Product id = 30044
Application Number = 20713
Date of Application = 22,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = MIRCETTE
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = TEVA BRANDED PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.15MG,N/A;0.02MG,0.01MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 29987
Application Number = 21090
Date of Application = 20,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = CYCLESSA
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = ORGANON USA INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.1MG,0.125MG,0.15MG;0.025MG,0.025MG,0.025MG
----
Product id = 29908
Application Number = 75256
Date of Application = 12,, Aug, 1999
RX/OTC/DISCN = DISCN
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-21 / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29993
Application Number = 75256
Date of Application = 12,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 30018
Application Number = 75863
Date of Application = 5,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = KARIVA
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.15MG,N/A;0.02MG,0.01MG
----
Product id = 30127
Application Number = 76455
Date of Application = 24,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = VELIVET
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG,0.125MG,0.15MG;0.025MG,0.025MG,0.025MG
----
Product id = 30003
Application Number = 76675
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = EMOQUETTE
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29998
Application Number = 76915
Date of Application = 29,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29996
Application Number = 76916
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG,N/A;0.02MG,0.01MG
----
Product id = 29997
Application Number = 77182
Date of Application = 24,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG,0.125MG,0.15MG;0.025MG,0.025MG,0.025MG
----
Product id = 29995
Application Number = 91234
Date of Application = 12,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = NOVAST LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 30104
Application Number = 91247
Date of Application = 1,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = PIMTREA
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = NOVAST LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG,N/A;0.02MG,0.01MG
----
Product id = 30128
Application Number = 91346
Date of Application = 2,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = VIORELE
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG,N/A;0.02MG,0.01MG
----
Product id = 30005
Application Number = 201887
Date of Application = 7,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = ENSKYCE
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG;0.03MG
----
Product id = 29994
Application Number = 202296
Date of Application = 30,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = DESOGESTREL AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = FAMY CARE LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.15MG,N/A;0.02MG,0.01MG
----