cyclophosphamide
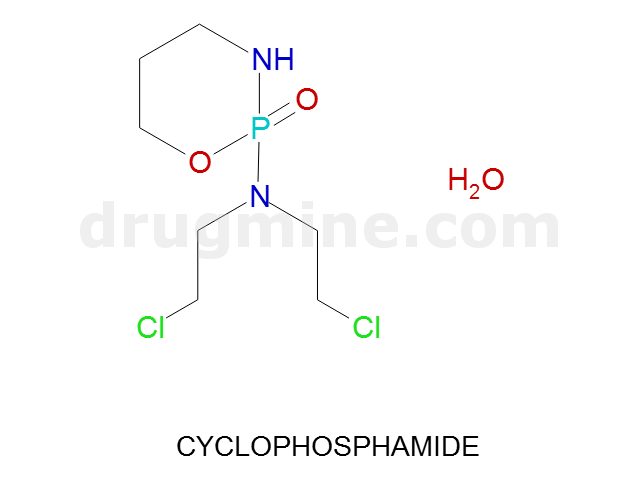
Name: CYCLOPHOSPHAMIDE
ID :
MW: 261
Number of atoms: 14
Molecular_Formula: C7H15Cl2N2O2P
Alogp: 0.144
Indication class : Immunosuppressant; Antineoplastic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
cyclophosphamide containing products summary
There are in total 36 different products containing the active ingredient cyclophosphamide. From the 36 drug products, 23 have been discontinued.Product id = 20112
Application Number = 12141
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20111
Application Number = 12141
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 6932
Application Number = 12142
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 6933
Application Number = 12142
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG/VIAL
----
Product id = 6938
Application Number = 12142
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 500MG/VIAL
----
Product id = 6934
Application Number = 12142
Date of Application = 30,, Aug, 1982
RX/OTC/DISCN = RX
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = 1GM/VIAL
----
Product id = 6936
Application Number = 12142
Date of Application = 30,, Aug, 1982
RX/OTC/DISCN = RX
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode = AP
Rld = Yes
Strength = 2GM/VIAL
----
Product id = 8978
Application Number = 12142
Date of Application = 5,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = LYOPHILIZED CYTOXAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 006
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 8979
Application Number = 12142
Date of Application = 10,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = LYOPHILIZED CYTOXAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 007
Tecode =
Rld = No
Strength = 200MG/VIAL
----
Product id = 6939
Application Number = 12142
Date of Application = 4,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 008
Tecode =
Rld = No
Strength = 500MG/VIAL
----
Product id = 6937
Application Number = 12142
Date of Application = 10,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 009
Tecode =
Rld = No
Strength = 2GM/VIAL
----
Product id = 6935
Application Number = 12142
Date of Application = 24,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = CYTOXAN (LYOPHILIZED)
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 010
Tecode =
Rld = No
Strength = 1GM/VIAL
----
Product id = 9627
Application Number = 40015
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 9628
Application Number = 40015
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG/VIAL
----
Product id = 9629
Application Number = 40015
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG/VIAL
----
Product id = 9625
Application Number = 40015
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 004
Tecode =
Rld = No
Strength = 1GM/VIAL
----
Product id = 9626
Application Number = 40015
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 005
Tecode =
Rld = No
Strength = 2GM/VIAL
----
Product id = 20075
Application Number = 40032
Date of Application = 17,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20076
Application Number = 40032
Date of Application = 17,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 50MG
----
Product id = 6901
Application Number = 40745
Date of Application = 21,, May, 2008
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 500MG/VIAL
----
Product id = 6896
Application Number = 40745
Date of Application = 21,, May, 2008
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 1GM/VIAL
----
Product id = 6898
Application Number = 40745
Date of Application = 21,, May, 2008
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 2GM/VIAL
----
Product id = 9622
Application Number = 87442
Date of Application = 16,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 9623
Application Number = 87442
Date of Application = 16,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG/VIAL
----
Product id = 9624
Application Number = 87442
Date of Application = 16,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG/VIAL
----
Product id = 9620
Application Number = 87442
Date of Application = 8,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 004
Tecode =
Rld = No
Strength = 1GM/VIAL
----
Product id = 9621
Application Number = 87442
Date of Application = 30,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = NEOSAR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 005
Tecode =
Rld = No
Strength = 2GM/VIAL
----
Product id = 6899
Application Number = 88371
Date of Application = 3,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 6900
Application Number = 88372
Date of Application = 3,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/VIAL
----
Product id = 6902
Application Number = 88373
Date of Application = 3,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/VIAL
----
Product id = 6897
Application Number = 88374
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM/VIAL
----
Product id = 1538
Application Number = 203856
Date of Application = 16,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 1539
Application Number = 203856
Date of Application = 16,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 50MG
----
Product id = 6905
Application Number = 204555
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = JIANGSU HENGRUI MED
ProductNo = 001
Tecode = AP
Rld = No
Strength = 500MG/VIAL
----
Product id = 6903
Application Number = 204555
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = JIANGSU HENGRUI MED
ProductNo = 002
Tecode = AP
Rld = No
Strength = 1GM/VIAL
----
Product id = 6904
Application Number = 204555
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = CYCLOPHOSPHAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = JIANGSU HENGRUI MED
ProductNo = 003
Tecode = AP
Rld = No
Strength = 2GM/VIAL
----