cortisone-acetate
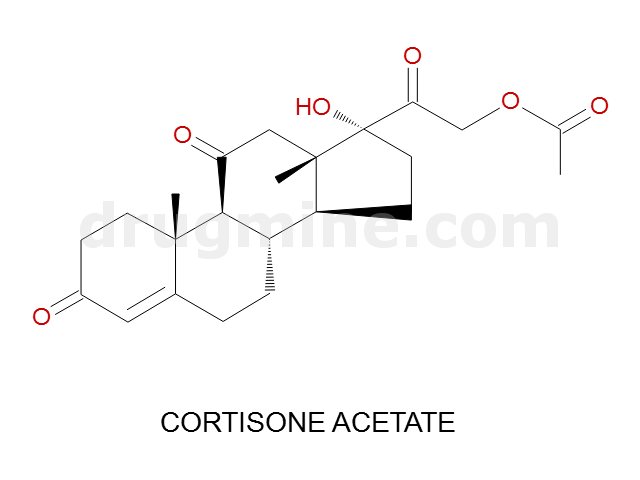

Name: CORTISONE ACETATE
ID :
MW: 402
Number of atoms: 29
Molecular_Formula: C23H30O6
Alogp: 1.619
Indication class : Glucocorticoid
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
cortisone acetate containing products summary
There are in total 27 different products containing the active ingredient cortisone acetate. From the 27 drug products, 26 have been discontinued.Product id = 6856
Application Number = 7110
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 6857
Application Number = 7110
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 20016
Application Number = 7750
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20011
Application Number = 8126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 6851
Application Number = 8126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 20009
Application Number = 8126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG
----
Product id = 20010
Application Number = 8126
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 004
Tecode =
Rld = No
Strength = 10MG
----
Product id = 20008
Application Number = 8284
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20007
Application Number = 8284
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PANRAY
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 20002
Application Number = 9458
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20013
Application Number = 80333
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VITARINE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20015
Application Number = 80341
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20012
Application Number = 80493
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20004
Application Number = 80630
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20006
Application Number = 80694
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20003
Application Number = 80731
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20001
Application Number = 80776
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 25MG
----
Product id = 19998
Application Number = 80836
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 6852
Application Number = 83147
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 6854
Application Number = 83147
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 19997
Application Number = 83471
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20005
Application Number = 83536
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19999
Application Number = 84246
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EVERYLIFE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 6853
Application Number = 85677
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 6855
Application Number = 85677
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 20000
Application Number = 85736
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HEATHER
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20014
Application Number = 85884
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CORTISONE ACETATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----