colchicine
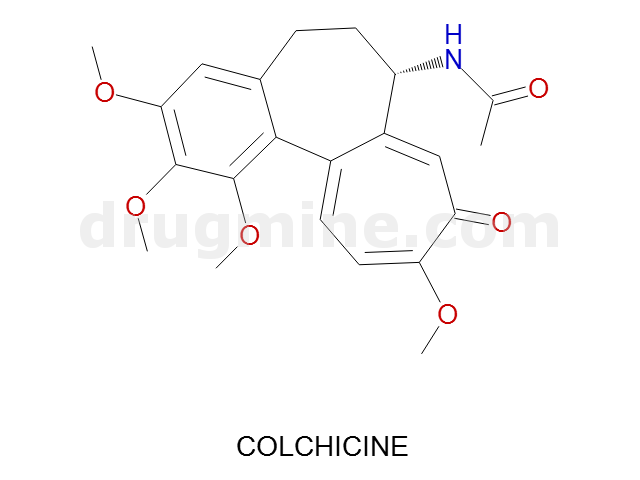


Name: COLCHICINE
ID :
MW: 399
Number of atoms: 29
Molecular_Formula: C22H25NO6
Alogp: 2.044
Indication class : Suppressant (gout)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
colchicine containing products summary
There are in total 12 different products containing the active ingredient colchicine. From the 12 drug products, 8 have been discontinued.Product id = 19962
Application Number = 12383
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = COLBENEMID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 19963
Application Number = 22352
Date of Application = 29,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = COLCRYS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.6MG
----
Product id = 26711
Application Number = 40618
Date of Application = 13,, May, 2008
RX/OTC/DISCN = RX
Tradename = PROBENECID AND COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIRROR PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26714
Application Number = 83221
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID W/ COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26709
Application Number = 83720
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID AND COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26710
Application Number = 83734
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID AND COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 19961
Application Number = 84279
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = COL-PROBENECID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.5MG;500MG
----
Product id = 26708
Application Number = 84321
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID AND COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BEECHAM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26702
Application Number = 85552
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBEN-C
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26712
Application Number = 86130
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID AND COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 26713
Application Number = 86954
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROBENECID W/ COLCHICINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;500MG
----
Product id = 2538
Application Number = 204820
Date of Application = 26,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = MITIGARE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6MG
----