clotrimazole
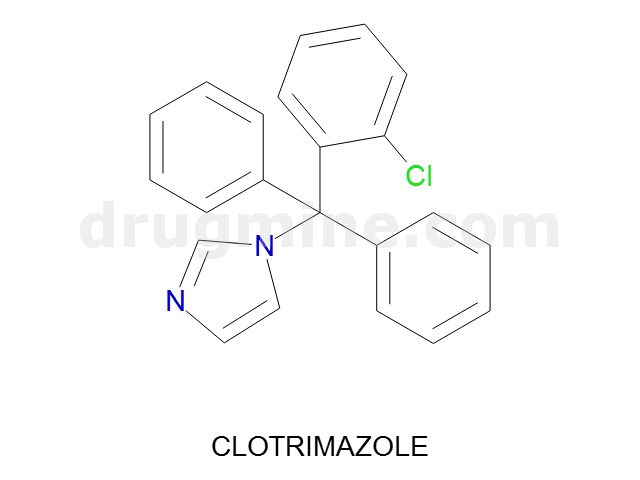

Name: CLOTRIMAZOLE
ID :
MW: 345
Number of atoms: 25
Molecular_Formula: C22H17ClN2
Alogp: 5.222
Indication class : Antifungal
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
clotrimazole containing products summary
There are in total 34 different products containing the active ingredient clotrimazole. From the 34 drug products, 8 have been discontinued.Product id = 14234
Application Number = 17613
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LOTRIMIN
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 4297
Application Number = 17619
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LOTRIMIN
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 30223
Application Number = 17717
Date of Application = 30,, Nov, 1990
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN
Route/format = VAGINAL / TABLET
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 100MG
----
Product id = 4481
Application Number = 18052
Date of Application = 30,, Nov, 1990
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1%
----
Product id = 14257
Application Number = 18181
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MYCELEX
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 30227
Application Number = 18182
Date of Application = 26,, Dec, 1991
RX/OTC/DISCN = OTC
Tradename = MYCELEX-7
Route/format = VAGINAL / TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 4318
Application Number = 18183
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MYCELEX
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 4494
Application Number = 18230
Date of Application = 26,, Dec, 1991
RX/OTC/DISCN = OTC
Tradename = MYCELEX-7
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 1%
----
Product id = 30256
Application Number = 18713
Date of Application = 17,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = MYCELEX
Route/format = ORAL / TROCHE/LOZENGE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 12230
Application Number = 18813
Date of Application = 17,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = LOTRIMIN
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 4299
Application Number = 18827
Date of Application = 10,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = LOTRISONE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE;1%
----
Product id = 30228
Application Number = 19069
Date of Application = 19,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCELEX-G
Route/format = VAGINAL / TABLET
Application Type = N
Applicant Name = BAYER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 12231
Application Number = 20010
Date of Application = 8,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = LOTRISONE
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE;1%
----
Product id = 4017
Application Number = 20289
Date of Application = 26,, Apr, 1993
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN COMBINATION PACK
Route/format = TOPICAL, VAGINAL / CREAM, TABLET
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1%,100MG
----
Product id = 4018
Application Number = 20389
Date of Application = 23,, Jun, 1994
RX/OTC/DISCN = OTC
Tradename = MYCELEX-7 COMBINATION PACK
Route/format = TOPICAL, VAGINAL / CREAM, TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 1%,100MG
----
Product id = 30224
Application Number = 20525
Date of Application = 29,, Jul, 1996
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN 3
Route/format = VAGINAL / TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 4016
Application Number = 20526
Date of Application = 29,, Jul, 1996
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN 3 COMBINATION PACK
Route/format = TOPICAL, VAGINAL / CREAM, TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1%,200MG
----
Product id = 4482
Application Number = 20574
Date of Application = 24,, Nov, 1998
RX/OTC/DISCN = OTC
Tradename = GYNE-LOTRIMIN 3
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2%
----
Product id = 4508
Application Number = 21143
Date of Application = 12,, Apr, 2000
RX/OTC/DISCN = OTC
Tradename = TRIVAGIZOLE 3
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 2%
----
Product id = 4095
Application Number = 72640
Date of Application = 31,, Aug, 1993
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1%
----
Product id = 4473
Application Number = 72641
Date of Application = 4,, Dec, 1995
RX/OTC/DISCN = OTC
Tradename = CLOTRIMAZOLE
Route/format = VAGINAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 30225
Application Number = 73249
Date of Application = 13,, Feb, 1998
RX/OTC/DISCN = DISCN
Tradename = GYNIX
Route/format = VAGINAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14156
Application Number = 73306
Date of Application = 28,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 4472
Application Number = 74165
Date of Application = 16,, Jul, 1993
RX/OTC/DISCN = OTC
Tradename = CLOTRIMAZOLE
Route/format = VAGINAL / CREAM
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----
Product id = 14155
Application Number = 74580
Date of Application = 29,, Jul, 1996
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 1%
----
Product id = 4097
Application Number = 75502
Date of Application = 5,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4098
Application Number = 75673
Date of Application = 29,, May, 2001
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4096
Application Number = 76002
Date of Application = 2,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 30249
Application Number = 76387
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = ORAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 12178
Application Number = 76493
Date of Application = 28,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 12177
Application Number = 76516
Date of Application = 16,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 30248
Application Number = 76763
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = ORAL / TROCHE/LOZENGE
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 4093
Application Number = 78338
Date of Application = 2,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1%
----
Product id = 4094
Application Number = 90219
Date of Application = 3,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = GLENMARK PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1%
----