clonazepam
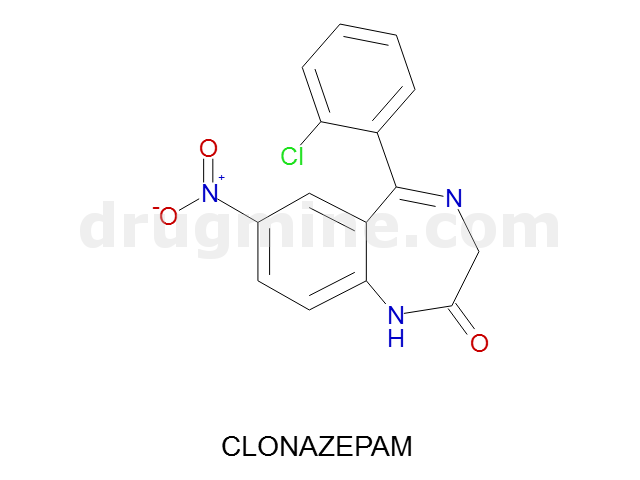
Name: CLONAZEPAM
ID :
MW: 316
Number of atoms: 22
Molecular_Formula: C15H10ClN3O3
Alogp: 2.859
Indication class : Anticonvulsant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
clonazepam containing products summary
There are in total 61 different products containing the active ingredient clonazepam. From the 61 drug products, 16 have been discontinued.Product id = 23183
Application Number = 17533
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KLONOPIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 23184
Application Number = 17533
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KLONOPIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 23185
Application Number = 17533
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KLONOPIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 23181
Application Number = 17533
Date of Application = 9,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 005
Tecode =
Rld = No
Strength = 0.125MG
----
Product id = 23182
Application Number = 17533
Date of Application = 9,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 006
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 16910
Application Number = 20813
Date of Application = 23,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN RAPIDLY DISINTEGRATING
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.125MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16911
Application Number = 20813
Date of Application = 23,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN RAPIDLY DISINTEGRATING
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16912
Application Number = 20813
Date of Application = 23,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN RAPIDLY DISINTEGRATING
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16913
Application Number = 20813
Date of Application = 23,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN RAPIDLY DISINTEGRATING
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ROCHE
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16914
Application Number = 20813
Date of Application = 23,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = KLONOPIN RAPIDLY DISINTEGRATING
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ROCHE
ProductNo = 005
Tecode =
Rld = No
Strength = 2MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 19790
Application Number = 74569
Date of Application = 10,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19792
Application Number = 74569
Date of Application = 10,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19794
Application Number = 74569
Date of Application = 10,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19769
Application Number = 74869
Date of Application = 31,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19770
Application Number = 74869
Date of Application = 31,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19771
Application Number = 74869
Date of Application = 31,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19791
Application Number = 74920
Date of Application = 4,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 19793
Application Number = 74920
Date of Application = 4,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 19795
Application Number = 74920
Date of Application = 4,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 2MG
----
Product id = 19781
Application Number = 74925
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 19783
Application Number = 74925
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 19785
Application Number = 74925
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 2MG
----
Product id = 19778
Application Number = 74940
Date of Application = 30,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19779
Application Number = 74940
Date of Application = 30,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19780
Application Number = 74940
Date of Application = 30,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19799
Application Number = 74964
Date of Application = 30,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19800
Application Number = 74964
Date of Application = 30,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19801
Application Number = 74964
Date of Application = 30,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19782
Application Number = 74979
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19784
Application Number = 74979
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19786
Application Number = 74979
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19775
Application Number = 75150
Date of Application = 5,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19776
Application Number = 75150
Date of Application = 5,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19777
Application Number = 75150
Date of Application = 5,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19787
Application Number = 75423
Date of Application = 27,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19788
Application Number = 75423
Date of Application = 27,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19789
Application Number = 75423
Date of Application = 27,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19772
Application Number = 75468
Date of Application = 6,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 19773
Application Number = 75468
Date of Application = 6,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 19774
Application Number = 75468
Date of Application = 6,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode =
Rld = No
Strength = 2MG
----
Product id = 19766
Application Number = 77147
Date of Application = 2,, May, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19767
Application Number = 77147
Date of Application = 2,, May, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19768
Application Number = 77147
Date of Application = 2,, May, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 16878
Application Number = 77171
Date of Application = 3,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.125MG
----
Product id = 16879
Application Number = 77171
Date of Application = 3,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 16880
Application Number = 77171
Date of Application = 3,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 16881
Application Number = 77171
Date of Application = 3,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 16882
Application Number = 77171
Date of Application = 3,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 16873
Application Number = 77194
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.125MG
----
Product id = 16874
Application Number = 77194
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 16875
Application Number = 77194
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 16876
Application Number = 77194
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = BARR
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 16877
Application Number = 77194
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = BARR
ProductNo = 005
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 19796
Application Number = 77856
Date of Application = 28,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 19797
Application Number = 77856
Date of Application = 28,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 19798
Application Number = 77856
Date of Application = 28,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 16883
Application Number = 78654
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.125MG
----
Product id = 16884
Application Number = 78654
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 16885
Application Number = 78654
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 16886
Application Number = 78654
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 16887
Application Number = 78654
Date of Application = 27,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CLONAZEPAM
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 2MG
----