clavulanate-potassium
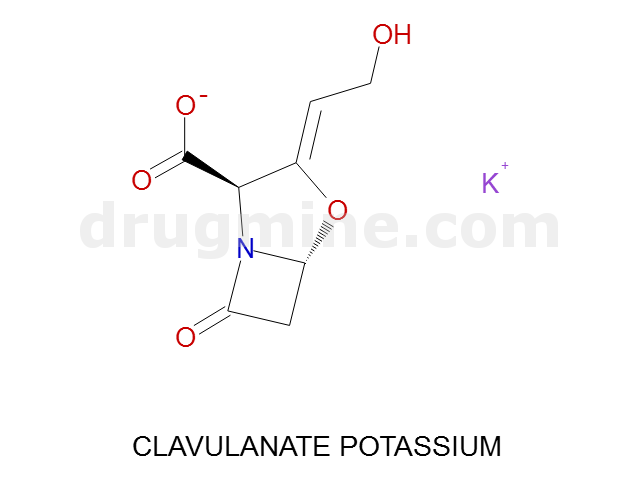

Name: CLAVULANATE POTASSIUM
ID :
MW: 198
Number of atoms: 14
Molecular_Formula: C8H8NO5
Alogp: -2.709
Indication class : Inhibitor (beta-lactamase)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
clavulanate potassium containing products summary
There are in total 59 different products containing the active ingredient clavulanate potassium. From the 59 drug products, 4 have been discontinued.Product id = 18214
Application Number = 50564
Date of Application = 6,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '250'
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 250MG;EQ 125MG BASE
----
Product id = 18215
Application Number = 50564
Date of Application = 6,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '500'
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 500MG;EQ 125MG BASE
----
Product id = 5055
Application Number = 50575
Date of Application = 6,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '125'
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG/5ML;EQ 31.25MG BASE/5ML
----
Product id = 5057
Application Number = 50575
Date of Application = 6,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '250'
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 250MG/5ML;EQ 62.5MG BASE/5ML
----
Product id = 11089
Application Number = 50590
Date of Application = 1,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = TIMENTIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 100MG BASE/VIAL;EQ 3GM BASE/VIAL
----
Product id = 11090
Application Number = 50590
Date of Application = 1,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = TIMENTIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 200MG BASE/VIAL;EQ 3GM BASE/VIAL
----
Product id = 11087
Application Number = 50590
Date of Application = 18,, Aug, 1987
RX/OTC/DISCN = RX
Tradename = TIMENTIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 1GM BASE/VIAL;EQ 30GM BASE/VIAL
----
Product id = 15217
Application Number = 50597
Date of Application = 22,, Jul, 1985
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '125'
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG;EQ 31.25MG BASE
----
Product id = 15219
Application Number = 50597
Date of Application = 22,, Jul, 1985
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '250'
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 250MG;EQ 62.5MG BASE
----
Product id = 11091
Application Number = 50658
Date of Application = 15,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = TIMENTIN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 100MG BASE/100ML;EQ 3GM BASE/100ML
----
Product id = 18216
Application Number = 50720
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '875'
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 875MG;EQ 125MG BASE
----
Product id = 5056
Application Number = 50725
Date of Application = 31,, May, 1996
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '200'
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 5058
Application Number = 50725
Date of Application = 31,, May, 1996
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '400'
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 15218
Application Number = 50726
Date of Application = 31,, May, 1996
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '200'
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG;EQ 28.5MG BASE
----
Product id = 15220
Application Number = 50726
Date of Application = 31,, May, 1996
RX/OTC/DISCN = RX
Tradename = AUGMENTIN '400'
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG;EQ 57MG BASE
----
Product id = 5059
Application Number = 50755
Date of Application = 22,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = AUGMENTIN ES-600
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 15719
Application Number = 50785
Date of Application = 25,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = AUGMENTIN XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1GM;EQ 62.5MG BASE
----
Product id = 11088
Application Number = 62691
Date of Application = 19,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = TIMENTIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE/VIAL;EQ 3GM BASE/VIAL
----
Product id = 17981
Application Number = 65063
Date of Application = 14,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 17980
Application Number = 65064
Date of Application = 15,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 15208
Application Number = 65065
Date of Application = 18,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG;EQ 28.5MG BASE
----
Product id = 15209
Application Number = 65065
Date of Application = 18,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG;EQ 57MG BASE
----
Product id = 5024
Application Number = 65066
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 5025
Application Number = 65066
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 5029
Application Number = 65089
Date of Application = 25,, May, 2004
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 5030
Application Number = 65089
Date of Application = 25,, May, 2004
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 17983
Application Number = 65093
Date of Application = 21,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 17985
Application Number = 65096
Date of Application = 29,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 5026
Application Number = 65098
Date of Application = 16,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 5027
Application Number = 65098
Date of Application = 16,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 17984
Application Number = 65101
Date of Application = 30,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 17977
Application Number = 65102
Date of Application = 17,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 17978
Application Number = 65109
Date of Application = 4,, Nov, 2002
RX/OTC/DISCN = DISCN
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 17982
Application Number = 65117
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 5021
Application Number = 65132
Date of Application = 19,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 5022
Application Number = 65132
Date of Application = 19,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 15206
Application Number = 65161
Date of Application = 3,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG;EQ 28.5MG BASE
----
Product id = 15207
Application Number = 65161
Date of Application = 3,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG;EQ 57MG BASE
----
Product id = 5031
Application Number = 65162
Date of Application = 12,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 17979
Application Number = 65189
Date of Application = 23,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG;EQ 125MG BASE
----
Product id = 5019
Application Number = 65191
Date of Application = 25,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 5018
Application Number = 65191
Date of Application = 25,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 15210
Application Number = 65205
Date of Application = 9,, Feb, 2005
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG;EQ 28.5MG BASE
----
Product id = 15211
Application Number = 65205
Date of Application = 9,, Feb, 2005
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 400MG;EQ 57MG BASE
----
Product id = 5023
Application Number = 65207
Date of Application = 30,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 17973
Application Number = 65317
Date of Application = 20,, Oct, 2008
RX/OTC/DISCN = DISCN
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode =
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 17971
Application Number = 65333
Date of Application = 24,, Feb, 2009
RX/OTC/DISCN = DISCN
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG;EQ 125MG BASE
----
Product id = 17972
Application Number = 65333
Date of Application = 24,, Feb, 2009
RX/OTC/DISCN = DISCN
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 5028
Application Number = 65358
Date of Application = 13,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 5020
Application Number = 65373
Date of Application = 9,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 5033
Application Number = 65420
Date of Application = 2,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = WOCKHARDT EU OPERATN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----
Product id = 5032
Application Number = 65431
Date of Application = 25,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG/5ML;EQ 62.5MG BASE/5ML
----
Product id = 15714
Application Number = 90227
Date of Application = 21,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1GM;EQ 62.5MG BASE
----
Product id = 17976
Application Number = 91568
Date of Application = 20,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 875MG;EQ 125MG BASE
----
Product id = 17974
Application Number = 91569
Date of Application = 20,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG;EQ 125MG BASE
----
Product id = 17975
Application Number = 91569
Date of Application = 20,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG;EQ 125MG BASE
----
Product id = 14637
Application Number = 201090
Date of Application = 20,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML;EQ 28.5MG BASE/5ML
----
Product id = 14638
Application Number = 201090
Date of Application = 20,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG/5ML;EQ 57MG BASE/5ML
----
Product id = 14639
Application Number = 201091
Date of Application = 20,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = AMOXICILLIN AND CLAVULANATE POTASSIUM
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG/5ML;EQ 42.9MG BASE/5ML
----