ciprofloxacin
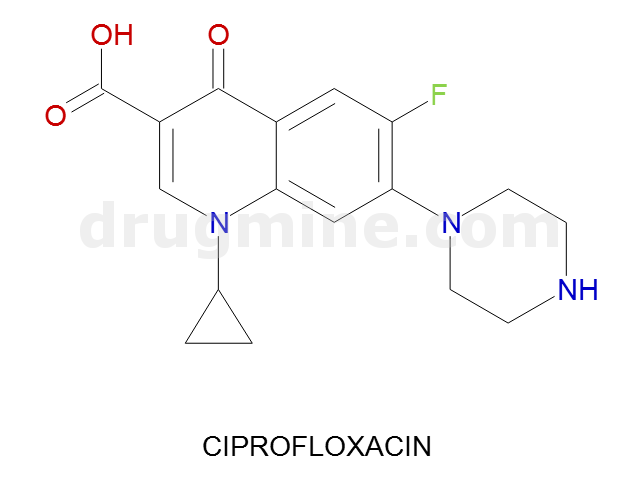
Name: CIPROFLOXACIN
ID :
MW: 331
Number of atoms: 24
Molecular_Formula: C17H18FN3O3
Alogp: -1.27
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ciprofloxacin containing products summary
There are in total 49 different products containing the active ingredient ciprofloxacin. From the 49 drug products, 20 have been discontinued.Product id = 6677
Application Number = 19847
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 400MG/40ML (10MG/ML)
----
Product id = 6678
Application Number = 19847
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6679
Application Number = 19847
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = CIPRO
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 1200MG/120ML (10MG/ML)
----
Product id = 6680
Application Number = 19857
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = CIPRO IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 200MG/100ML
----
Product id = 6681
Application Number = 19857
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = CIPRO IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 400MG/200ML
----
Product id = 6682
Application Number = 19858
Date of Application = 26,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = CIPRO IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAYER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 5183
Application Number = 20780
Date of Application = 26,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG/5ML
----
Product id = 5184
Application Number = 20780
Date of Application = 26,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 500MG/5ML
----
Product id = 15814
Application Number = 21473
Date of Application = 13,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = CIPRO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15815
Application Number = 21473
Date of Application = 28,, Aug, 2003
RX/OTC/DISCN = DISCN
Tradename = CIPRO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 14595
Application Number = 21537
Date of Application = 18,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = CIPRODEX
Route/format = OTIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.3%;0.1%
----
Product id = 6688
Application Number = 76484
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6689
Application Number = 76484
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 6690
Application Number = 76717
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6691
Application Number = 76717
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 6696
Application Number = 76757
Date of Application = 21,, Apr, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 6683
Application Number = 76992
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6684
Application Number = 76992
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 6685
Application Number = 76993
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1200MG/120ML (10MG/ML)
----
Product id = 6708
Application Number = 77138
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 6709
Application Number = 77138
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML
----
Product id = 6692
Application Number = 77245
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6693
Application Number = 77245
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 15816
Application Number = 77417
Date of Application = 30,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15821
Application Number = 77701
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 15820
Application Number = 77701
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 6706
Application Number = 77753
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML
----
Product id = 6707
Application Number = 77753
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML
----
Product id = 6694
Application Number = 77782
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6695
Application Number = 77782
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 15817
Application Number = 77809
Date of Application = 30,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 6699
Application Number = 77888
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 6700
Application Number = 77888
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML
----
Product id = 6703
Application Number = 78024
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML
----
Product id = 6704
Application Number = 78024
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML
----
Product id = 6686
Application Number = 78062
Date of Application = 29,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/20ML (10MG/ML)
----
Product id = 6687
Application Number = 78062
Date of Application = 29,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/40ML (10MG/ML)
----
Product id = 6701
Application Number = 78114
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 6702
Application Number = 78114
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/200ML
----
Product id = 15819
Application Number = 78166
Date of Application = 27,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 15818
Application Number = 78166
Date of Application = 27,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15822
Application Number = 78183
Date of Application = 22,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15823
Application Number = 78183
Date of Application = 22,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 6697
Application Number = 78252
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR INFO SA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 200MG/100ML
----
Product id = 6698
Application Number = 78252
Date of Application = 18,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR INFO SA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 400MG/200ML
----
Product id = 6705
Application Number = 78431
Date of Application = 18,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 400MG/200ML
----
Product id = 15824
Application Number = 78712
Date of Application = 11,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 5185
Application Number = 200563
Date of Application = 5,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG/5ML
----
Product id = 5186
Application Number = 200563
Date of Application = 5,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG/5ML
----