cidofovir
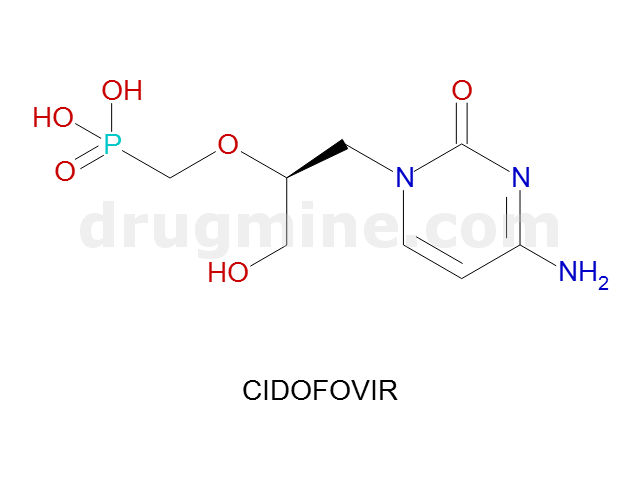
Name: CIDOFOVIR
ID :
MW: 279
Number of atoms: 18
Molecular_Formula: C8H14N3O6P
Alogp: -2.175
Indication class : Antiviral
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cidofovir containing products summary
There are in total 3 different products containing the active ingredient cidofovir. From the 3 drug products, zero have been discontinued.Product id = 11411
Application Number = 20638
Date of Application = 26,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = VISTIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GILEAD SCIENCES INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 75MG BASE/ML
----
Product id = 6659
Application Number = 201276
Date of Application = 27,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = CIDOFOVIR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 75MG BASE/ML
----
Product id = 6658
Application Number = 202501
Date of Application = 26,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = CIDOFOVIR
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 75MG BASE/ML
----