choline-fenofibrate

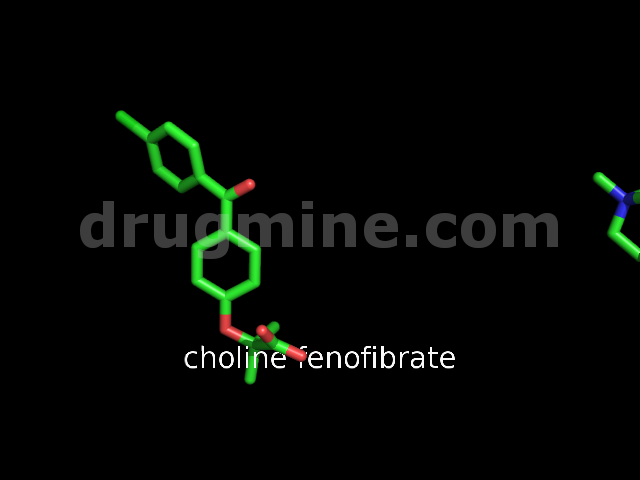
Name: CHOLINE FENOFIBRATE
ID :
MW: 318
Number of atoms: 22
Molecular_Formula: C17H14ClO4
Alogp: 2.685
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
choline fenofibrate containing products summary
There are in total 8 different products containing the active ingredient choline fenofibrate. From the 8 drug products, zero have been discontinued.Product id = 286
Application Number = 22224
Date of Application = 15,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = TRILIPIX
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 45MG FENOFIBRIC ACID
----
Product id = 287
Application Number = 22224
Date of Application = 15,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = TRILIPIX
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 135MG FENOFIBRIC ACID
----
Product id = 266
Application Number = 200750
Date of Application = 4,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 45MG FENOFIBRIC ACID
----
Product id = 267
Application Number = 200750
Date of Application = 4,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 135MG FENOFIBRIC ACID
----
Product id = 268
Application Number = 200913
Date of Application = 25,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 45MG FENOFIBRIC ACID
----
Product id = 269
Application Number = 200913
Date of Application = 25,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 135MG FENOFIBRIC ACID
----
Product id = 265
Application Number = 201573
Date of Application = 18,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 135MG FENOFIBRIC ACID
----
Product id = 264
Application Number = 201573
Date of Application = 18,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = FENOFIBRIC ACID
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 45MG FENOFIBRIC ACID
----