chlorpromazine-hydrochloride
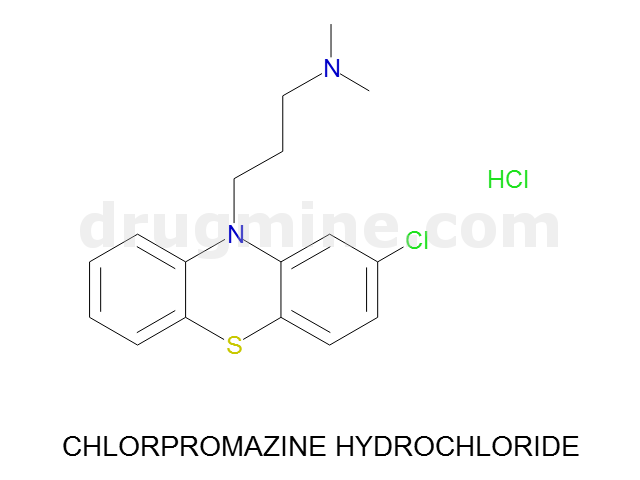
Name: CHLORPROMAZINE HYDROCHLORIDE
ID :
MW: 319
Number of atoms: 21
Molecular_Formula: C17H19ClN2S
Alogp: 4.739
Indication class : Antipsychotic; Anti-Emetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
chlorpromazine hydrochloride containing products summary
There are in total 92 different products containing the active ingredient chlorpromazine hydrochloride. From the 92 drug products, 81 have been discontinued.Product id = 28689
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 28690
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 007
Tecode =
Rld = No
Strength = 25MG
----
Product id = 11075
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 011
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 28691
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 013
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28692
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 018
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28693
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 020
Tecode =
Rld = No
Strength = 200MG
----
Product id = 15158
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 022
Tecode =
Rld = No
Strength = 10MG/5ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 3995
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CONCENTRATE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 032
Tecode =
Rld = No
Strength = 30MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 3996
Application Number = 9149
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CONCENTRATE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 043
Tecode =
Rld = No
Strength = 100MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 794
Application Number = 11120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 016
Tecode =
Rld = No
Strength = 30MG
----
Product id = 795
Application Number = 11120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 017
Tecode =
Rld = No
Strength = 75MG
----
Product id = 796
Application Number = 11120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 018
Tecode =
Rld = No
Strength = 150MG
----
Product id = 797
Application Number = 11120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 019
Tecode =
Rld = No
Strength = 200MG
----
Product id = 798
Application Number = 11120
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THORAZINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 020
Tecode =
Rld = No
Strength = 300MG
----
Product id = 3910
Application Number = 40224
Date of Application = 26,, Jan, 1999
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 3909
Application Number = 40231
Date of Application = 30,, Dec, 1999
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 19367
Application Number = 80340
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19368
Application Number = 80340
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19369
Application Number = 80340
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 003
Tecode =
Rld = No
Strength = 200MG
----
Product id = 6627
Application Number = 80365
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 6629
Application Number = 80370
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 19363
Application Number = 80403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19364
Application Number = 80403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19365
Application Number = 80403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19362
Application Number = 80403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19366
Application Number = 80403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 005
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19375
Application Number = 80439
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = BP
Rld = No
Strength = 10MG
----
Product id = 19376
Application Number = 80439
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = BP
Rld = No
Strength = 25MG
----
Product id = 19377
Application Number = 80439
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = BP
Rld = No
Strength = 50MG
----
Product id = 19378
Application Number = 80439
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = BP
Rld = Yes
Strength = 100MG
----
Product id = 19379
Application Number = 80439
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode = BP
Rld = No
Strength = 200MG
----
Product id = 3969
Application Number = 80983
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SONAZINE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 3970
Application Number = 80983
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SONAZINE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 15148
Application Number = 83040
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SONAZINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 6625
Application Number = 83329
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 25MG/ML
----
Product id = 19380
Application Number = 83386
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = BP
Rld = No
Strength = 10MG
----
Product id = 19347
Application Number = 83549
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19348
Application Number = 83549
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19349
Application Number = 83549
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19350
Application Number = 83574
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19351
Application Number = 83575
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19381
Application Number = 84112
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = BP
Rld = No
Strength = 25MG
----
Product id = 19382
Application Number = 84113
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = BP
Rld = No
Strength = 50MG
----
Product id = 19383
Application Number = 84114
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = BP
Rld = No
Strength = 100MG
----
Product id = 19384
Application Number = 84115
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = BP
Rld = No
Strength = 200MG
----
Product id = 19344
Application Number = 84411
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19345
Application Number = 84412
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19346
Application Number = 84413
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19342
Application Number = 84414
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19343
Application Number = 84415
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 26758
Application Number = 84423
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMAPAR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19360
Application Number = 84789
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19359
Application Number = 84800
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19358
Application Number = 84801
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19361
Application Number = 84802
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19357
Application Number = 84803
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 6624
Application Number = 84911
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 19370
Application Number = 85331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19371
Application Number = 85331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19372
Application Number = 85331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19373
Application Number = 85331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19374
Application Number = 85331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 005
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19354
Application Number = 85484
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 6628
Application Number = 85591
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 19356
Application Number = 85748
Date of Application = 4,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KV PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19352
Application Number = 85750
Date of Application = 4,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KV PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19353
Application Number = 85751
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19355
Application Number = 85752
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19389
Application Number = 85956
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19391
Application Number = 85957
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19392
Application Number = 85958
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19388
Application Number = 85959
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19390
Application Number = 85960
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 14961
Application Number = 86712
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/5ML
----
Product id = 3908
Application Number = 86863
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 26761
Application Number = 86885
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMAPAR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 26757
Application Number = 86886
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMAPAR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26759
Application Number = 86887
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMAPAR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 26760
Application Number = 86888
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMAPAR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 3911
Application Number = 87032
Date of Application = 8,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 3912
Application Number = 87053
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 19386
Application Number = 87645
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19387
Application Number = 87646
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19393
Application Number = 87783
Date of Application = 16,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 19394
Application Number = 87865
Date of Application = 16,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 19395
Application Number = 87878
Date of Application = 15,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 19397
Application Number = 87880
Date of Application = 16,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 19396
Application Number = 87884
Date of Application = 15,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 19385
Application Number = 88038
Date of Application = 16,, Aug, 1982
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 3913
Application Number = 88157
Date of Application = 27,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE INTENSOL
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG/ML
----
Product id = 3914
Application Number = 88158
Date of Application = 27,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE INTENSOL
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 6626
Application Number = 89563
Date of Application = 15,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = CHLORPROMAZINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----