carisoprodol
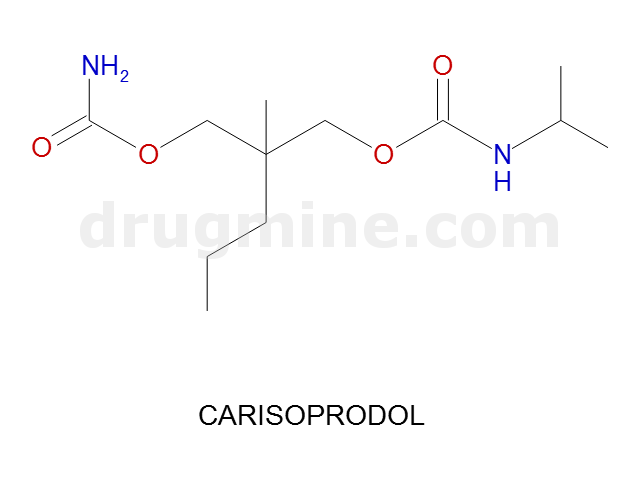

Name: CARISOPRODOL
ID :
MW: 260
Number of atoms: 18
Molecular_Formula: C12H24N2O4
Alogp: 2.199
Indication class : Relaxant (skeletal muscle)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
carisoprodol containing products summary
There are in total 34 different products containing the active ingredient carisoprodol. From the 34 drug products, 15 have been discontinued.Product id = 28100
Application Number = 11792
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SOMA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 3300
Application Number = 11792
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SOMA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 28099
Application Number = 11792
Date of Application = 13,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = SOMA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 004
Tecode =
Rld = Yes
Strength = 250MG
----
Product id = 27398
Application Number = 12155
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RELA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 28101
Application Number = 12365
Date of Application = 11,, Jul, 1983
RX/OTC/DISCN = RX
Tradename = SOMA COMPOUND
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 325MG;200MG
----
Product id = 28102
Application Number = 12366
Date of Application = 11,, Jul, 1983
RX/OTC/DISCN = RX
Tradename = SOMA COMPOUND W/ CODEINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 325MG;200MG;16MG
----
Product id = 19036
Application Number = 40116
Date of Application = 25,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL AND ASPIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;200MG
----
Product id = 19040
Application Number = 40118
Date of Application = 16,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;200MG;16MG
----
Product id = 19017
Application Number = 40124
Date of Application = 24,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19029
Application Number = 40152
Date of Application = 3,, Dec, 1996
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19023
Application Number = 40188
Date of Application = 7,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19028
Application Number = 40245
Date of Application = 8,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19035
Application Number = 40252
Date of Application = 10,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL AND ASPIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;200MG
----
Product id = 19039
Application Number = 40283
Date of Application = 29,, Dec, 1998
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 325MG;200MG;16MG
----
Product id = 19016
Application Number = 40397
Date of Application = 21,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19013
Application Number = 40421
Date of Application = 21,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19014
Application Number = 40576
Date of Application = 7,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCELRX LABS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19027
Application Number = 40755
Date of Application = 27,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19015
Application Number = 40792
Date of Application = 6,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19018
Application Number = 40823
Date of Application = 22,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIRROR PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19034
Application Number = 40832
Date of Application = 7,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL AND ASPIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIRROR PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;200MG
----
Product id = 19038
Application Number = 40860
Date of Application = 7,, Jan, 2010
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MIRROR PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 325MG;200MG;16MG
----
Product id = 19024
Application Number = 81025
Date of Application = 13,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19030
Application Number = 85433
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19031
Application Number = 86179
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19032
Application Number = 87499
Date of Application = 20,, Apr, 1982
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19037
Application Number = 88809
Date of Application = 3,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL COMPOUND
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 325MG;200MG
----
Product id = 19019
Application Number = 89346
Date of Application = 17,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19022
Application Number = 89390
Date of Application = 13,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PIONEER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19025
Application Number = 89566
Date of Application = 30,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 350MG
----
Product id = 19033
Application Number = 89594
Date of Application = 31,, Mar, 1989
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL AND ASPIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 325MG;200MG
----
Product id = 19020
Application Number = 90988
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19026
Application Number = 203374
Date of Application = 27,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----
Product id = 19021
Application Number = 205085
Date of Application = 28,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = CARISOPRODOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORIENT PHARMA CO LTD
ProductNo = 001
Tecode = AA
Rld = No
Strength = 350MG
----