buprenorphine-hydrochloride
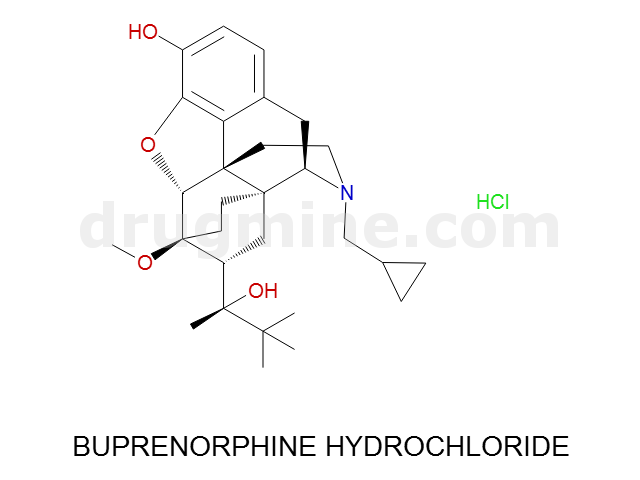
Name: BUPRENORPHINE HYDROCHLORIDE
ID :
MW: 468
Number of atoms: 34
Molecular_Formula: C29H41NO4
Alogp: 3.923
Indication class : Analgesic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
buprenorphine hydrochloride containing products summary
There are in total 37 different products containing the active ingredient buprenorphine hydrochloride. From the 37 drug products, 4 have been discontinued.Product id = 6215
Application Number = 18401
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = BUPRENEX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 0.3MG BASE/ML
----
Product id = 30215
Application Number = 20732
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBUTEX
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30216
Application Number = 20732
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBUTEX
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 30213
Application Number = 20733
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBOXONE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30214
Application Number = 20733
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBOXONE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 4801
Application Number = 22410
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 4803
Application Number = 22410
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 4802
Application Number = 22410
Date of Application = 10,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 4MG BASE;EQ 1MG BASE
----
Product id = 4804
Application Number = 22410
Date of Application = 10,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 12MG BASE;EQ 3MG BASE
----
Product id = 6217
Application Number = 74137
Date of Application = 3,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.3MG BASE/ML
----
Product id = 6216
Application Number = 76931
Date of Application = 2,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.3MG BASE/ML
----
Product id = 6218
Application Number = 78331
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 0.3MG BASE/ML
----
Product id = 30153
Application Number = 78633
Date of Application = 8,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30154
Application Number = 78633
Date of Application = 8,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE
----
Product id = 30147
Application Number = 90360
Date of Application = 7,, May, 2010
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30148
Application Number = 90360
Date of Application = 7,, May, 2010
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 30149
Application Number = 90622
Date of Application = 24,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ETHYPHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30150
Application Number = 90622
Date of Application = 24,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ETHYPHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 30145
Application Number = 90819
Date of Application = 19,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30146
Application Number = 90819
Date of Application = 19,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 30160
Application Number = 91149
Date of Application = 8,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30161
Application Number = 91149
Date of Application = 8,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30155
Application Number = 91422
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30156
Application Number = 91422
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30151
Application Number = 201066
Date of Application = 6,, Mar, 2015
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 30152
Application Number = 201066
Date of Application = 6,, Mar, 2015
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 30162
Application Number = 203136
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30157
Application Number = 203136
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30158
Application Number = 203326
Date of Application = 27,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30159
Application Number = 203326
Date of Application = 27,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30218
Application Number = 204242
Date of Application = 3,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1.4MG BASE;EQ 0.36MG BASE
----
Product id = 30219
Application Number = 204242
Date of Application = 3,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 5.7MG BASE;EQ 1.4MG BASE
----
Product id = 30220
Application Number = 204242
Date of Application = 11,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 8.6MG BASE;EQ 2.1MG BASE
----
Product id = 30221
Application Number = 204242
Date of Application = 11,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 11.4MG BASE;EQ 2.9MG BASE
----
Product id = 4790
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2.1MG BASE;EQ 0.3MG BASE
----
Product id = 4791
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4.2MG BASE;EQ 0.7MG BASE
----
Product id = 4792
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 6.3MG BASE;EQ 1MG BASE
----