baclofen
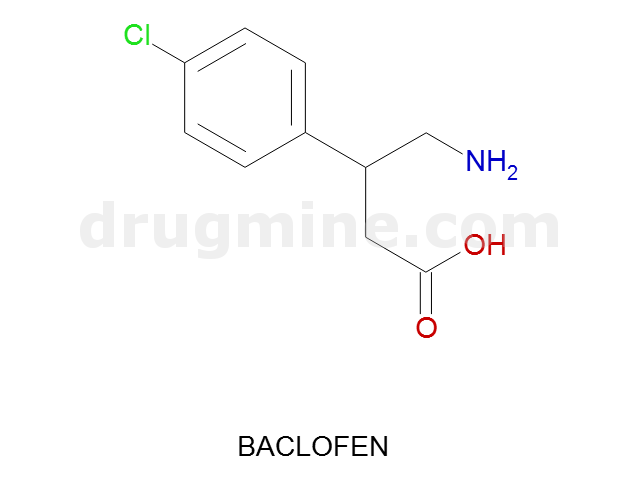
Name: BACLOFEN
ID :
MW: 214
Number of atoms: 14
Molecular_Formula: C10H12ClNO2
Alogp: -1.06
Indication class : Relaxant (muscle)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
baclofen containing products summary
There are in total 41 different products containing the active ingredient baclofen. From the 41 drug products, 12 have been discontinued.Product id = 23670
Application Number = 17851
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LIORESAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23671
Application Number = 17851
Date of Application = 20,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = LIORESAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 11613
Application Number = 20075
Date of Application = 17,, Jun, 1992
RX/OTC/DISCN = RX
Tradename = LIORESAL
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MEDTRONIC NEURO
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 0.5MG/ML
----
Product id = 11614
Application Number = 20075
Date of Application = 17,, Jun, 1992
RX/OTC/DISCN = RX
Tradename = LIORESAL
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MEDTRONIC NEURO
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 2MG/ML
----
Product id = 11612
Application Number = 20075
Date of Application = 7,, Nov, 1996
RX/OTC/DISCN = RX
Tradename = LIORESAL
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MEDTRONIC NEURO
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 0.05MG/ML
----
Product id = 16908
Application Number = 21589
Date of Application = 30,, Oct, 2003
RX/OTC/DISCN = DISCN
Tradename = KEMSTRO
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16909
Application Number = 21589
Date of Application = 30,, Oct, 2003
RX/OTC/DISCN = DISCN
Tradename = KEMSTRO
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = UCB INC
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 11607
Application Number = 22462
Date of Application = 19,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = GABLOFEN
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.05MG/ML
----
Product id = 11608
Application Number = 22462
Date of Application = 19,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = GABLOFEN
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = 0.5MG/ML
----
Product id = 11610
Application Number = 22462
Date of Application = 19,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = GABLOFEN
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 11609
Application Number = 22462
Date of Application = 22,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = GABLOFEN
Route/format = INTRATHECAL / INJECTABLE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 18301
Application Number = 71260
Date of Application = 6,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18303
Application Number = 71261
Date of Application = 6,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 18286
Application Number = 72234
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18287
Application Number = 72235
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 18307
Application Number = 72824
Date of Application = 18,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18310
Application Number = 72825
Date of Application = 18,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18299
Application Number = 73043
Date of Application = 27,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18300
Application Number = 73044
Date of Application = 27,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 18308
Application Number = 73092
Date of Application = 28,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18311
Application Number = 73093
Date of Application = 28,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 18302
Application Number = 74584
Date of Application = 19,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18304
Application Number = 74584
Date of Application = 19,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18309
Application Number = 74698
Date of Application = 20,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18312
Application Number = 74698
Date of Application = 20,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 18306
Application Number = 77068
Date of Application = 30,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18297
Application Number = 77088
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18296
Application Number = 77089
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18291
Application Number = 77121
Date of Application = 29,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18305
Application Number = 77156
Date of Application = 30,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18290
Application Number = 77181
Date of Application = 29,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18289
Application Number = 77241
Date of Application = 20,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18298
Application Number = 77862
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18285
Application Number = 77971
Date of Application = 26,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18283
Application Number = 77984
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARACO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18284
Application Number = 78146
Date of Application = 26,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18288
Application Number = 78220
Date of Application = 6,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18295
Application Number = 78401
Date of Application = 18,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18294
Application Number = 78504
Date of Application = 18,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18292
Application Number = 90334
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18293
Application Number = 90334
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = BACLOFEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----