atazanavir-sulfate
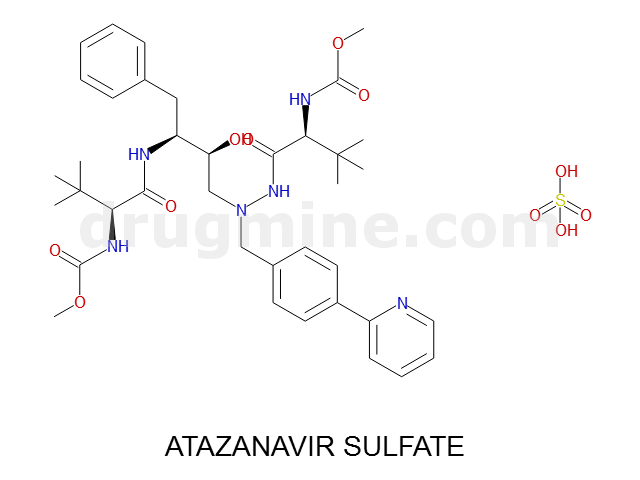
Name: ATAZANAVIR SULFATE
ID :
MW: 705
Number of atoms: 51
Molecular_Formula: C38H52N6O7
Alogp: 4.69
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
atazanavir sulfate containing products summary
There are in total 10 different products containing the active ingredient atazanavir sulfate. From the 10 drug products, 1 have been discontinued.Product id = 3176
Application Number = 21567
Date of Application = 20,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = REYATAZ
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 3177
Application Number = 21567
Date of Application = 20,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = REYATAZ
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 3178
Application Number = 21567
Date of Application = 20,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = REYATAZ
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 200MG BASE
----
Product id = 3179
Application Number = 21567
Date of Application = 16,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = REYATAZ
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 300MG BASE
----
Product id = 1103
Application Number = 91673
Date of Application = 22,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ATAZANAVIR SULFATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 1104
Application Number = 91673
Date of Application = 22,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ATAZANAVIR SULFATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 150MG BASE
----
Product id = 1105
Application Number = 91673
Date of Application = 22,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ATAZANAVIR SULFATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 200MG BASE
----
Product id = 1106
Application Number = 91673
Date of Application = 22,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ATAZANAVIR SULFATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 300MG BASE
----
Product id = 12775
Application Number = 206352
Date of Application = 2,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = REYATAZ
Route/format = ORAL / POWDER
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 50MG BASE/PACKET
----
Product id = 21124
Application Number = 206353
Date of Application = 29,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = EVOTAZ
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 300MG BASE;150MG
----