argatroban
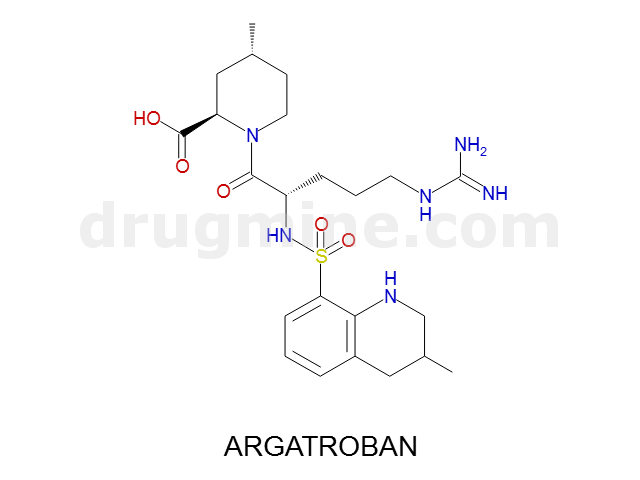
Name: ARGATROBAN
ID :
MW: 509
Number of atoms: 35
Molecular_Formula: C23H36N6O5S
Alogp: 1.266
Indication class : Anticoagulant
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
argatroban containing products summary
There are in total 8 different products containing the active ingredient argatroban. From the 8 drug products, 1 have been discontinued.Product id = 5612
Application Number = 20883
Date of Application = 30,, Jun, 2000
RX/OTC/DISCN = RX
Tradename = ACOVA
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 250MG/2.5ML (100MG/ML)
----
Product id = 11742
Application Number = 22434
Date of Application = 29,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = ARGATROBAN IN SODIUM CHLORIDE
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = EAGLE PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG/50ML (1MG/ML)
----
Product id = 11743
Application Number = 22485
Date of Application = 9,, May, 2011
RX/OTC/DISCN = RX
Tradename = ARGATROBAN IN SODIUM CHLORIDE
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = Yes
Strength = 125MG/125ML (1MG/ML)
----
Product id = 6008
Application Number = 91665
Date of Application = 30,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = ARGATROBAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PAR STERILE PRODUCTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 250MG/2.5ML (100MG/ML)
----
Product id = 13693
Application Number = 201743
Date of Application = 9,, May, 2011
RX/OTC/DISCN = DISCN
Tradename = ARGATROBAN IN DEXTROSE
Route/format = IV (INFUSION) / SOLUTION
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG/125ML (1MG/ML)
----
Product id = 6007
Application Number = 202626
Date of Application = 30,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = ARGATROBAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 250MG/2.5ML (100MG/ML)
----
Product id = 6006
Application Number = 203049
Date of Application = 5,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = ARGATROBAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA PHARM CO LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 250MG/2.5ML (100MG/ML)
----
Product id = 11741
Application Number = 206769
Date of Application = 15,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ARGATROBAN IN 0.9% SODIUM CHLORIDE
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG/250ML (1MG/ML)
----