anastrozole
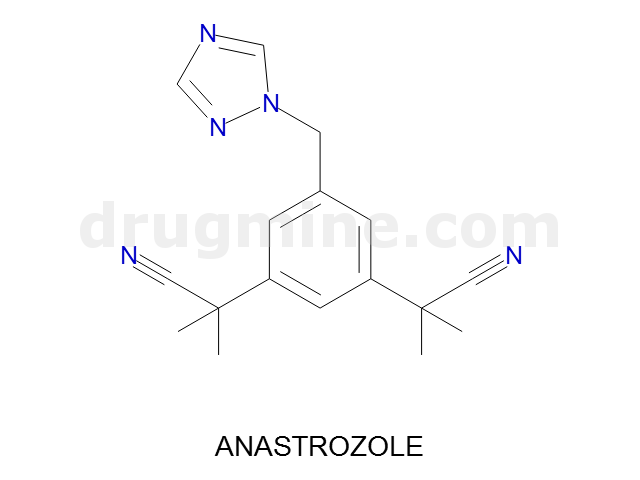
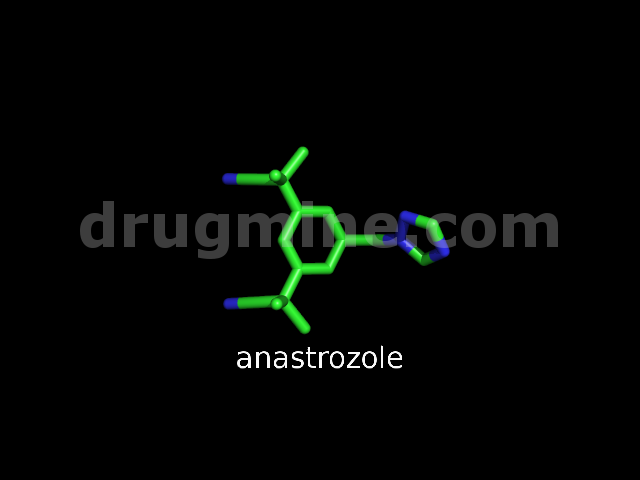
Name: ANASTROZOLE
ID :
MW: 293
Number of atoms: 22
Molecular_Formula: C17H19N5
Alogp: 2.965
Indication class : Antineoplastic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
anastrozole containing products summary
There are in total 18 different products containing the active ingredient anastrozole. From the 18 drug products, 4 have been discontinued.Product id = 18067
Application Number = 20541
Date of Application = 27,, Dec, 1995
RX/OTC/DISCN = RX
Tradename = ARIMIDEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 18017
Application Number = 78058
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18016
Application Number = 78322
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 18012
Application Number = 78485
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18019
Application Number = 78921
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18014
Application Number = 78944
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANTOS BIOTECH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18018
Application Number = 78984
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 18013
Application Number = 79007
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18011
Application Number = 79220
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18007
Application Number = 90088
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18003
Application Number = 90568
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18006
Application Number = 90732
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18010
Application Number = 91051
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18005
Application Number = 91164
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18015
Application Number = 91177
Date of Application = 15,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 18008
Application Number = 91242
Date of Application = 31,, May, 2012
RX/OTC/DISCN = DISCN
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 18009
Application Number = 91331
Date of Application = 5,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 18004
Application Number = 200654
Date of Application = 11,, May, 2012
RX/OTC/DISCN = RX
Tradename = ANASTROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----