anagrelide-hydrochloride
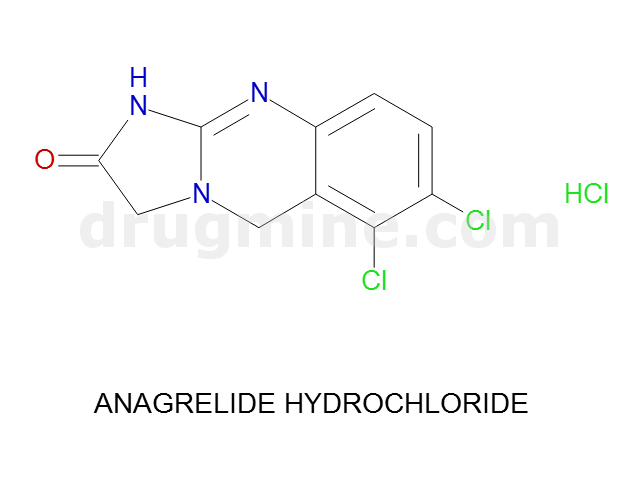
Name: ANAGRELIDE HYDROCHLORIDE
ID :
MW: 256
Number of atoms: 16
Molecular_Formula: C10H7Cl2N3O
Alogp: 1.965
Indication class : Antithrombotic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
anagrelide hydrochloride containing products summary
There are in total 18 different products containing the active ingredient anagrelide hydrochloride. From the 18 drug products, 7 have been discontinued.Product id = 945
Application Number = 20333
Date of Application = 14,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = AGRYLIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 946
Application Number = 20333
Date of Application = 14,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = AGRYLIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1080
Application Number = 76417
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1081
Application Number = 76417
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1070
Application Number = 76468
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1071
Application Number = 76468
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 1MG BASE
----
Product id = 1076
Application Number = 76489
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1077
Application Number = 76489
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1066
Application Number = 76530
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1067
Application Number = 76530
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1078
Application Number = 76683
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1079
Application Number = 76683
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1072
Application Number = 76811
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1073
Application Number = 76811
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1068
Application Number = 76910
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1069
Application Number = 76910
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 1074
Application Number = 77613
Date of Application = 27,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE
----
Product id = 1075
Application Number = 77613
Date of Application = 27,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = ANAGRELIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----