amoxapine
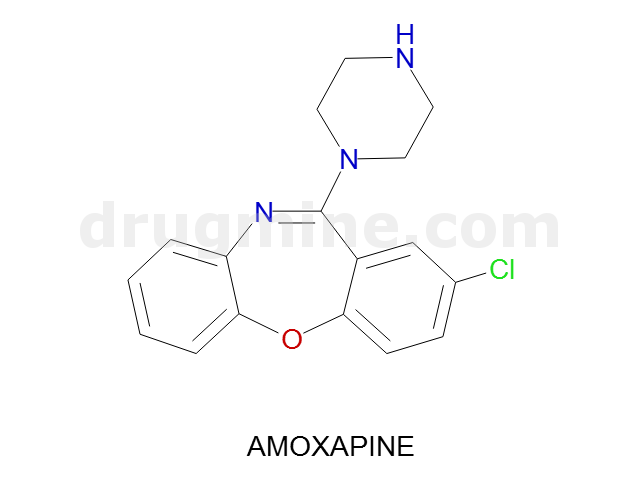

Name: AMOXAPINE
ID :
MW: 314
Number of atoms: 22
Molecular_Formula: C17H16ClN3O
Alogp: 2.897
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
amoxapine containing products summary
There are in total 16 different products containing the active ingredient amoxapine. From the 16 drug products, 12 have been discontinued.Product id = 18082
Application Number = 18021
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ASENDIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18083
Application Number = 18021
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ASENDIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEDERLE
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18084
Application Number = 18021
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ASENDIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEDERLE
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18085
Application Number = 18021
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ASENDIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEDERLE
ProductNo = 004
Tecode =
Rld = No
Strength = 150MG
----
Product id = 17953
Application Number = 72418
Date of Application = 11,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 17955
Application Number = 72419
Date of Application = 11,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 17957
Application Number = 72420
Date of Application = 11,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 17959
Application Number = 72421
Date of Application = 11,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 17954
Application Number = 72688
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 17956
Application Number = 72689
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 17958
Application Number = 72690
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 17960
Application Number = 72691
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 150MG
----
Product id = 17951
Application Number = 72878
Date of Application = 28,, Jun, 1991
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 17952
Application Number = 72879
Date of Application = 28,, Jun, 1991
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 17949
Application Number = 72943
Date of Application = 28,, Jun, 1991
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 17950
Application Number = 72944
Date of Application = 28,, Jun, 1991
RX/OTC/DISCN = DISCN
Tradename = AMOXAPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----