alendronate-sodium
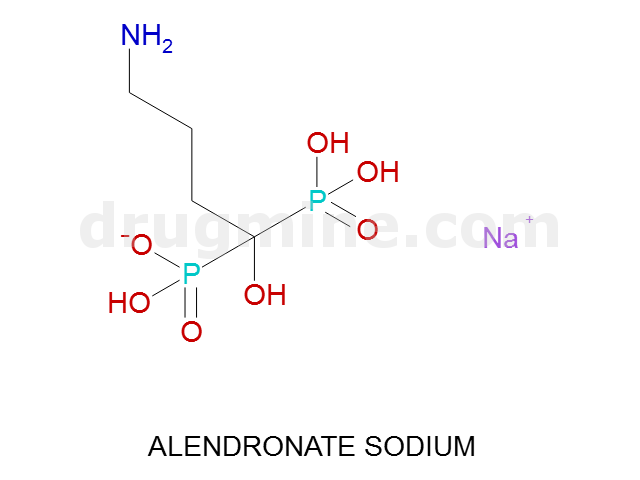

Name: ALENDRONATE SODIUM
ID :
MW: 248
Number of atoms: 14
Molecular_Formula: C4H12NO7P2
Alogp: -2.677
Indication class : Bone Resorption Inhibitor
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
alendronate sodium containing products summary
There are in total 59 different products containing the active ingredient alendronate sodium. From the 59 drug products, 13 have been discontinued.Product id = 21551
Application Number = 20560
Date of Application = 29,, Sep, 1995
RX/OTC/DISCN = DISCN
Tradename = FOSAMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK AND CO INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 21553
Application Number = 20560
Date of Application = 29,, Sep, 1995
RX/OTC/DISCN = DISCN
Tradename = FOSAMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK AND CO INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 40MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 21550
Application Number = 20560
Date of Application = 25,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = FOSAMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK AND CO INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 5MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 21552
Application Number = 20560
Date of Application = 20,, Oct, 2000
RX/OTC/DISCN = DISCN
Tradename = FOSAMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK AND CO INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 35MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 21554
Application Number = 20560
Date of Application = 20,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = FOSAMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK AND CO INC
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = EQ 70MG BASE
----
Product id = 13817
Application Number = 21575
Date of Application = 17,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = FOSAMAX
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 70MG BASE/75ML
----
Product id = 21555
Application Number = 21762
Date of Application = 7,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = FOSAMAX PLUS D
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 70MG BASE;2,800 IU
----
Product id = 21556
Application Number = 21762
Date of Application = 26,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = FOSAMAX PLUS D
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 70MG BASE;5,600 IU
----
Product id = 17479
Application Number = 75710
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17480
Application Number = 75710
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17481
Application Number = 75710
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17483
Application Number = 75710
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 17484
Application Number = 75710
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17470
Application Number = 75871
Date of Application = 22,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17471
Application Number = 75871
Date of Application = 22,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17473
Application Number = 75871
Date of Application = 22,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 17472
Application Number = 75871
Date of Application = 22,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17474
Application Number = 75871
Date of Application = 22,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17485
Application Number = 76184
Date of Application = 6,, Feb, 2008
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17482
Application Number = 76184
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17464
Application Number = 76584
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17465
Application Number = 76584
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17466
Application Number = 76584
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17468
Application Number = 76584
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17455
Application Number = 76768
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17456
Application Number = 76768
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17457
Application Number = 76768
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17458
Application Number = 76768
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 17459
Application Number = 76768
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17486
Application Number = 76984
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17487
Application Number = 76984
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 17488
Application Number = 76984
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17440
Application Number = 77982
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17441
Application Number = 77982
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17442
Application Number = 77982
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17443
Application Number = 77982
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17467
Application Number = 78638
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17469
Application Number = 78638
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17462
Application Number = 79049
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17463
Application Number = 79049
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17460
Application Number = 79109
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17461
Application Number = 79109
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17475
Application Number = 90022
Date of Application = 10,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17476
Application Number = 90022
Date of Application = 10,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17477
Application Number = 90022
Date of Application = 10,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17478
Application Number = 90022
Date of Application = 10,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17444
Application Number = 90124
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17445
Application Number = 90124
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17446
Application Number = 90124
Date of Application = 4,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 17447
Application Number = 90258
Date of Application = 24,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUSTARPHARMA LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17448
Application Number = 90258
Date of Application = 24,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUSTARPHARMA LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17449
Application Number = 90258
Date of Application = 24,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUSTARPHARMA LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17450
Application Number = 90258
Date of Application = 24,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUSTARPHARMA LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 13735
Application Number = 90520
Date of Application = 25,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 70MG BASE/75ML
----
Product id = 17451
Application Number = 90557
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17452
Application Number = 90557
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17453
Application Number = 90557
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 35MG BASE
----
Product id = 17454
Application Number = 90557
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ALENDRONATE SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 70MG BASE
----
Product id = 15615
Application Number = 202344
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = BINOSTO
Route/format = ORAL / TABLET, EFFERVESCENT
Application Type = N
Applicant Name = MISSION PHARMA
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 70MG BASE
----