acyclovir
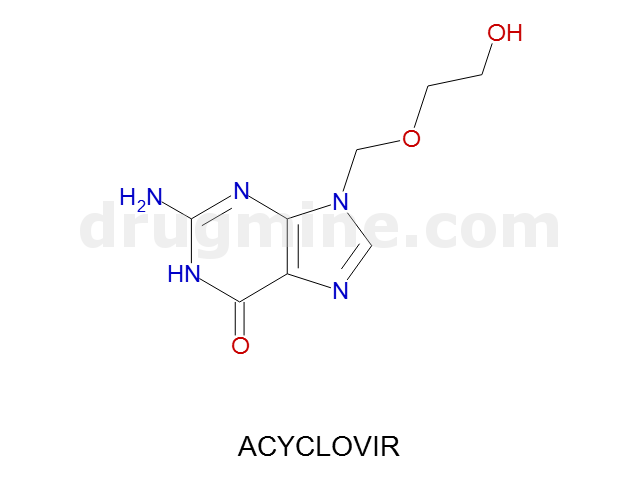
Name: ACYCLOVIR
ID :
MW: 225
Number of atoms: 16
Molecular_Formula: C8H11N5O3
Alogp: -1.423
Indication class : Antiviral
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
acyclovir containing products summary
There are in total 62 different products containing the active ingredient acyclovir. From the 62 drug products, 25 have been discontinued.Product id = 12615
Application Number = 18604
Date of Application = 29,, Mar, 1982
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5%
----
Product id = 3904
Application Number = 18828
Date of Application = 25,, Jan, 1985
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 14841
Application Number = 19909
Date of Application = 22,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG/5ML
----
Product id = 29872
Application Number = 20089
Date of Application = 30,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 29873
Application Number = 20089
Date of Application = 30,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 800MG
----
Product id = 4459
Application Number = 21478
Date of Application = 30,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = ZOVIRAX
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5%
----
Product id = 4457
Application Number = 22436
Date of Application = 31,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = XERESE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5%;1%
----
Product id = 17361
Application Number = 74556
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 17362
Application Number = 74556
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17363
Application Number = 74556
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 929
Application Number = 74570
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 931
Application Number = 74578
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 17353
Application Number = 74658
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEK PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17354
Application Number = 74658
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEK PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 924
Application Number = 74674
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 926
Application Number = 74727
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 14633
Application Number = 74738
Date of Application = 28,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML
----
Product id = 925
Application Number = 74750
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LEK PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 932
Application Number = 74828
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 921
Application Number = 74833
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 17343
Application Number = 74834
Date of Application = 24,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17345
Application Number = 74834
Date of Application = 24,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 17351
Application Number = 74836
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17352
Application Number = 74836
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 17335
Application Number = 74870
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17336
Application Number = 74870
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 922
Application Number = 74872
Date of Application = 22,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 923
Application Number = 74889
Date of Application = 31,, Oct, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 17347
Application Number = 74891
Date of Application = 31,, Oct, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17348
Application Number = 74891
Date of Application = 31,, Oct, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 918
Application Number = 74906
Date of Application = 26,, Aug, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 933
Application Number = 74914
Date of Application = 26,, Nov, 1997
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 17344
Application Number = 74946
Date of Application = 19,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17346
Application Number = 74946
Date of Application = 19,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 928
Application Number = 74975
Date of Application = 30,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 17355
Application Number = 74976
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17357
Application Number = 74976
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 927
Application Number = 74977
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 17359
Application Number = 74980
Date of Application = 30,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17360
Application Number = 74980
Date of Application = 30,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 17364
Application Number = 75021
Date of Application = 18,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 17365
Application Number = 75021
Date of Application = 18,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 800MG
----
Product id = 930
Application Number = 75090
Date of Application = 26,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = STASON
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 934
Application Number = 75101
Date of Application = 15,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 17356
Application Number = 75211
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17358
Application Number = 75211
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 17341
Application Number = 75382
Date of Application = 30,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17342
Application Number = 75382
Date of Application = 30,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 919
Application Number = 75677
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 14634
Application Number = 77026
Date of Application = 7,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG/5ML
----
Product id = 17337
Application Number = 77309
Date of Application = 29,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17338
Application Number = 77309
Date of Application = 29,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 920
Application Number = 201445
Date of Application = 6,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CADILA PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 17339
Application Number = 202168
Date of Application = 15,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADILA PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17340
Application Number = 202168
Date of Application = 15,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADILA PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 12385
Application Number = 202459
Date of Application = 3,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5%
----
Product id = 17137
Application Number = 203791
Date of Application = 12,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = SITAVIG
Route/format = BUCCAL / TABLET
Application Type = N
Applicant Name = INNOCUTIS HOLDINGS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG
----
Product id = 17349
Application Number = 203834
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HETERO LABS LTD V
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17350
Application Number = 203834
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HETERO LABS LTD V
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 17366
Application Number = 204314
Date of Application = 19,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 17367
Application Number = 204314
Date of Application = 19,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 800MG
----
Product id = 12384
Application Number = 204605
Date of Application = 18,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = ACYCLOVIR
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5%
----