acetazolamide-sodium
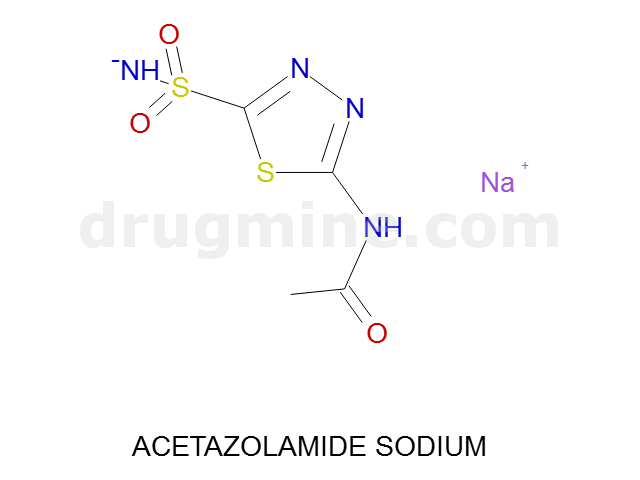

Name: ACETAZOLAMIDE SODIUM
ID :
MW: 221
Number of atoms: 13
Molecular_Formula: C4H5N4O3S2
Alogp: -1.226
Indication class : .
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
acetazolamide sodium containing products summary
There are in total 6 different products containing the active ingredient acetazolamide sodium. From the 6 drug products, 2 have been discontinued.Product id = 7192
Application Number = 9388
Date of Application = 5,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = DIAMOX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 5603
Application Number = 40089
Date of Application = 28,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = ACETAZOLAMIDE SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 5604
Application Number = 40108
Date of Application = 30,, Oct, 1995
RX/OTC/DISCN = DISCN
Tradename = ACETAZOLAMIDE SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 5606
Application Number = 40784
Date of Application = 10,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = ACETAZOLAMIDE SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = X GEN PHARMS
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 5605
Application Number = 200880
Date of Application = 9,, May, 2012
RX/OTC/DISCN = RX
Tradename = ACETAZOLAMIDE SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT AGILA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 5607
Application Number = 202693
Date of Application = 19,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ACETAZOLAMIDE SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----